Annex "Contaminants in bird eggs"
Methods
In order to study spatial patterns in bird pollution, sampling sites from the western to the northern parts of the Wadden Sea were chosen, including different habitats of the Wadden Sea coast (see Figure 1, for details see Becker et al., 2001). According to the QSR subarea coding of the Wadden Sea, bird eggs were collected in (1) Balgzand and (2) Griend (QSR code N1: Western Dutch Wadden Sea), (3) Julianapolder and (4) Schiermonnikoog (QSR code N2: Eastern Dutch Wadden Sea), (5) Delfzijl and (6) Dollard (QSR code N3: Ems-Dollard estuary, the first site in the Netherlands and the second one in Germany), (7) Baltrum (QSR code NDS1: Lower Saxon Wadden Sea), (8) Minsener Oog and (9) Mellum (QSR code NDS2: Jade basin), Neufelderkoog and Hullen (QSR code SH1: Elbe estuary), Trischen (QSR code SH2: Inner German Bight), Norderoog (until 2006) and Hallig Hooge (since 2007) (QSR code SH3: Halligen), Margrethekoog (QSR code D1: Sylt-Rømø basin), and Langli (QSR code D3: Varden and Sneum estuary). At sites 4 (since 2005), 7, 11 and 15 only common tern eggs were collected, at the sites 3 (since 2005), 6, 7, 9 and 16 only oystercatcher eggs were taken; at sites 1, 2, 3 (until 2004), 5, 12 and 13/14 eggs of both species were sampled. To determine contaminant levels, ten fresh eggs per species, site and year were collected if possible (one egg per clutch chosen randomly). Egg samples from Denmark (D1 and D3) were not collected each year due to the lack of financial resources.
All chemical analyses were made at ICBM-Terramare, Wilhelmshaven, Germany. For details about chemical procedures see Goutner et al. (2015). Concentrations of the following chemicals were determined: Mercury (Hg), SPCB (including 62 polychlorinated biphenyl congeners), hexachlorobenzene (HCB), SDDT (including p,p’-DDT, o,p’-DDT, o,p’-DDD, p,p’-DDD, o,p’-DDE, and p,p’-DDE), SHCH (including a-, b- and g-isomers of hexachlorocyclohexane) and Schlordane. Since 2000, the o,p’-isomers of DDT, DDD and DDE have been excluded, as they were no longer detected. Chlordanes have been included since 1998 and their levels are presented as SChlordane, summing up levels of cis- and trans-chlordane, and cis- and trans-nonachlor. The summed concentrations of ten dioxin-like PCBs were calculated, namely the non-ortho PCB congeners PCB126 and 169 and mono-ortho congeners PCB105, 114, 118, 123, 156, 157, 167 and 189 (e.g. Becker & Dittmann, 2009). The Toxic Equivalents (TEQs) of non- and mono-ortho congeners in eggs were calculated using bird-specific 2,3,7,8-TCDD toxic equivalency factors (TEF) proposed by the WHO (Van den Berg et al., 1998).
Mercury determination was carried out by atomic absorption spectrometry and organochlorines were determined by gas chromatography. The determination limit for mercury was 0.1 ng·g-1 and for organochlorines varied between 0.3-0.4 ng·g-1 for single organochlorines, and for p,p’-DDT it was 0.9 ng·g-1. The residue levels are given in ng g-1 fresh egg mass. In the figures, arithmetic means ± 95 % confidence intervals are shown. If the confidence intervals of two groups do not overlap, a significant difference of at least p<0.05 is given.
Environmental chemicals
From Wikipedia, the free encyclopedia, and from Mattig (1998).
PCB
A polychlorinated biphenyl (PCB) is an organic chlorine compound with the formula C12H10−xClx. The number of chlorine atoms ranges from one to ten per PCB molecule, so there could be 209 different PCB molecules, named congeners. Polychlorinated biphenyls were once widely used as dielectric or cooling fluids in some types of electrical apparatus, in heat transfer fluids and in carbonless copy paper. The produced PCBs were a mixture of different congeners with an unknown content. Normally only the mass percentage of chlorine in the mixture is indicated. From a technical point of view PCBs have many advantages (longevity, chemical-resistant, dielectric, flame-resistant etc.) so they were widely used. Because of the environmental toxicity of PCBs and their classification as a persistent organic pollutant, PCB production was banned worldwide by the Stockholm Convention on Persistent Organic Pollutants in 2001. The International Research Agency on Cancer (IRAC), rendered PCBs as definite carcinogens in humans. Many rivers and buildings including schools, parks, and other sites are contaminated with PCBs, and there have been contaminations of food with the toxins.
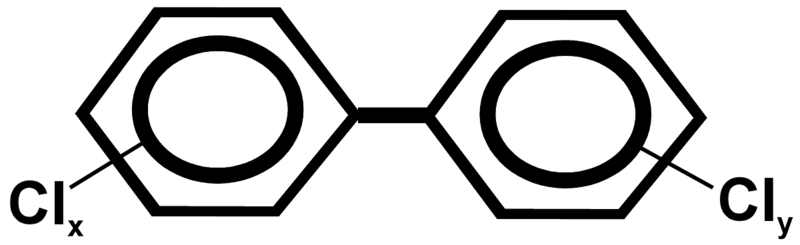
Figure A.1. Chemical structure of PCBs. The possible positions of chlorine atoms on the benzene rings are indicated (x = 1-5; y = 0-5).
Some PCBs share a structural similarity and toxic mode of action with dioxin. Other toxic effects such as endocrine disruption (notably blocking of thyroid system functioning) and neurotoxicity are known.
DDT
DDT (dichlorodiphenyltrichloroethane) is a colorless, crystalline, tasteless and almost odorless organochlorine known for its insecticidal properties and environmental impacts. DDT has been formulated in multiple forms, including solutions in xylene or petroleum distillates, emulsifiable concentrates, water-wettable powders, granules, aerosols, smoke candles and charges for vaporizers and lotions.
First synthesized in 1874, DDT's insecticidal action was discovered by the Swiss chemist Paul Hermann Müller in 1939. It was used in the second half of World War II to control malaria and typhus among civilians and troops. After the war, DDT was also used as an agricultural insecticide and its production and use increased accordingly. Müller was awarded the Nobel Prize in Physiology or Medicine "for his discovery of the high efficiency of DDT as a contact poison against several arthropods" in 1948.
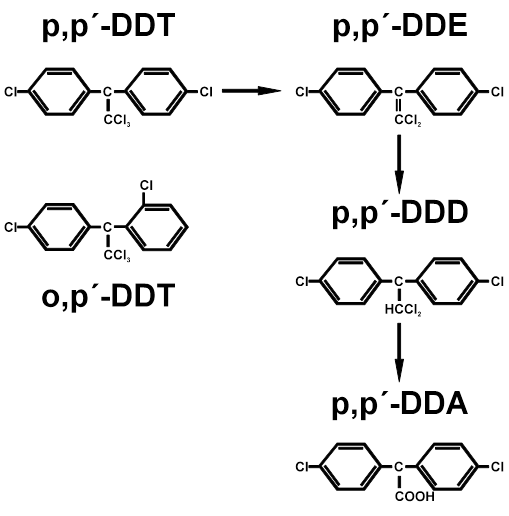
Figure A.2. Chemical structure of DDT and its metabolites.
In 1962, Rachel Carson's book Silent Spring was published. It catalogued the environmental impacts of widespread DDT spraying in the United States and questioned the logic of releasing large amounts of potentially dangerous chemicals into the environment without understanding their effects on the environment or human health. The book claimed that DDT and other pesticides had been shown to cause cancer and that their agricultural use was a threat to wildlife, particularly birds. Its publication was a seminal event for the environmental movement and resulted in a large public outcry that led to a worldwide ban on agricultural use, formalized under the Stockholm Convention on Persistent Organic Pollutants. But the limited and still controversial use of the substance in disease vector control continues because of its effectiveness in reducing malarial infections, balanced by environmental and other health concerns.
HCH
Hexachlorocyclohexane (HCH) is an organic chlorine compound with the formula C6H6Cl6 consisting of a six-carbon ring with one chlorine and one hydrogen atom attached to each carbon atom. There are many different isomers for this structure, differing in the stereochemistry of the individual chlorine substituents on the cyclohexane. Technical HCH is a mixture from the a-, b-, g-, d- and e‑HCH isomers, with a proportion of 15 % g-HCH, the only isomer with an insecticide activity. Lindan, with a proportion of 99 % g-HCH, was used as an agricultural insecticide. HCH is a persistent organic pollutant: it is relatively long-lived in the environment, it is transported over long distances by natural processes like the hydrologic cycle, and it can bioaccumulate in food chains. HCH is a neurotoxin that interferes with the transmembrane GABA (gamma-aminobutyric acid) receptor-chloride channel complex. GABA is the most important inhibitory neurotransmitter of the brain and the central nervous system. In humans, HCH affects the nervous system, liver and kidneys, and may well be carcinogenic. It is unclear whether it an endocrine disruptor. Therefore, in 2009, the production and agricultural use of HCH was banned under the Stockholm Convention on persistent organic pollutant.
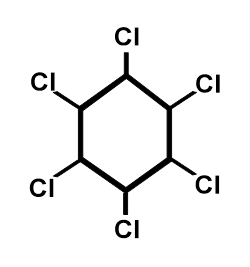
Figure A.3. Chemical structure of HCH.
HCB
Hexachlorobenzene (HCB) is an organochloride with the molecular formula C6Cl6. It is a fungicide formerly used in seed treatment, especially in wheat to control the fungal disease bunt. It has been banned globally under the Stockholm Convention on persistent organic pollutants.

Figure A.4. Chemical structure of HCB.
HCB is very toxic to aquatic organisms. It may cause long-term adverse effects in the aquatic environment. Therefore, release into waterways should be avoided. It is persistent in the environment. Ecological investigations have found that biomagnification up the food chain occurs. Hexachlorobenzene has a half-life in the soil of between 3 and 6 years. The risk of bioaccumulation in an aquatic species is high.
Chlordane
Chlordane, or chlordan, is an organochlorine compound used as a pesticide. This white solid was sold e.g. in the U.S. until 1988 as a contact insecticide for treating approximately 30 million homes for termite control, for use in crops like corn and citrus, as well as on lawns and in domestic gardens. Technical grade chlordane is a complex mixture of over 120 structurally related chemical compounds. Integral components were trans-Chlordan, cis-Chlordan, trans-Nonachlor and Heptachlor.
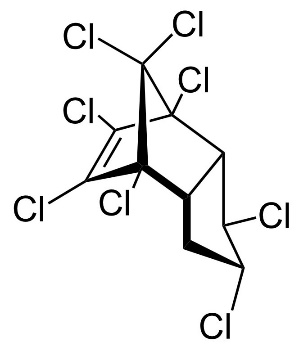 |
 |
 |
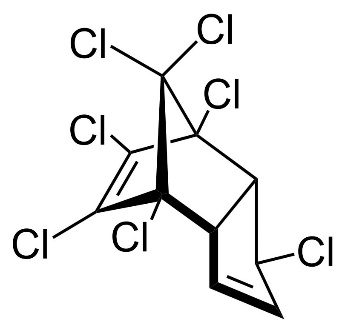 |
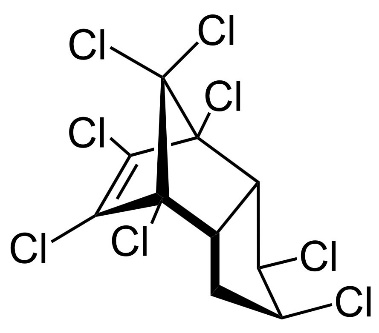
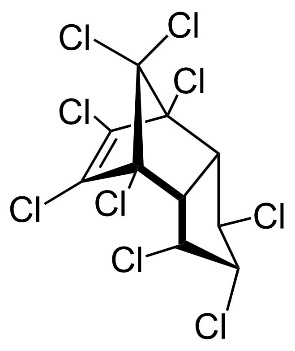
Figure 13. Chemical structure of trans-Chlordan, cis-Chlordan, trans-Nonachlor and Heptachlor (from left to right).
Chlordane is very persistent in the environment because it does not break down easily. It has been banned globally under the Stockholm Convention on persistent organic pollutants.
