Photo: Christian Buschbaum. Crepidulamytilus.
Alien species
H. Büttger, S. Christoph, C. Buschbaum, A. Gittenberger, K. Jensen, S. Kabuta, D. Lackschewitz
Published 2022
(Version 1.01)
1. Introduction
The spread of organisms is in principle a natural process, which is limited by natural geographical barriers. However, human activities have breached those barriers and have – intentionally and unintentionally – transferred species into areas beyond their natural range (Table 1). With intensified global trade, the spread of species outside their natural range was accelerated and now raises concern about biological homogenization (Capinha et al., 2015), threatening of native species and ecosystems, and human health and economic issues (Nehring et al., 2009). The anthropogenic introduction of alien species, in particular invasive ones, seriously violates the guiding principle of the trilateral Wadden Sea policy “to achieve, as far as possible, a natural and sustainable ecosystem in which natural processes proceed in an undisturbed way” (CWSS, 2010; see also Bouma et al. 2011). Most alien species became inconspicuous residents and cannot be removed without causing collateral damage to the ecosystem, and more alien species are likely to come (Seebens et al., 2017).
Many alien species have reached the Wadden Sea in the last decades (e.g., see Figure 1 and Annex). This may enhance diversity in the Wadden Sea but also cause biotic homogenization among regions (Reise et al., 2005; Nehring et al., 2009). There is as yet no evidence that alien species have expelled native organisms from the Wadden Sea (Wolff, 2000; Buschbaum et al., 2012). However, alien species have the potential to alter dominance structures, habitats, and trophic connections, like, e.g., the Pacific oyster Magallana gigas and the Japanese seaweed Sargassum muticum (Reise et al. 2005).
Major vectors of marine species introduction in the North Sea, respectively in the Wadden Sea, are shipping, with species travelling via ballast water and hull fouling, and species imports for aquaculture activities, with species travelling as hitchhikers (Figure 1, Nehring, 2002; Wolff & Reise, 2002; Buschbaum et al., 2012; Schuchardt & Sevilgen, 2015; van der Have et al., 2015). Hull fouling has been described as one of the main transport vectors (Nehring, 2002; Gittenberger et al., 2017), more recent publications state ballast water to be the most important transport vector for marine alien species worldwide, followed by hull fouling (Stranga & Katsanevakis, 2021). Marine alien species reached the Wadden Sea also through secondary dispersal from sites of primary introduction (Buschbaum et al., 2012).
For terrestrial and freshwater species main introduction vectors are horticulture, pet trade and transport (van der Have et al., 2015). Escapes, e.g., from zoos or gardens, can lead to spread and establishment in nature (van der Have et al., 2015; Zieritz et al., 2017; Nehring & Skowronek, 2020; McGrannachan et al., 2021). Nevertheless, it is often unknown or uncertain how an alien species has reached the Wadden Sea.
This thematic report reviews the status and trend of marine and terrestrial alien species in the Wadden Sea, assesses the effects of alien species on the Wadden Sea Plan targets and the criteria for the ‘Outstanding Universal Value’ (OUV) and gives recommendations for monitoring, research, and management.
 Figure 1: Examples of alien species in the Wadden Sea (clockwise starting with top left): The Pacific amphipod Aoroides semicurvatus was introduced with hull fouling and/or shellfish imports (Photo: Björn Nadarzinski, Labor für Meeresbiologie, Werkstätten Materialhof), the marine yellow-green algae Vaucheria cf. velutina probably introduced by transoceanic shipping or with shellfish translocation (Photo: Karsten Reise), the Egyptian goose Alopochen aegyptiaca intentionally introduced by zoo and pet trade (Photo: Armin Rose), and the racoon dog Nyctereutes procyonoides is an escapee from fur-farms (Photo: Michael Gäbler/ Wikimedia Commons).
Figure 1: Examples of alien species in the Wadden Sea (clockwise starting with top left): The Pacific amphipod Aoroides semicurvatus was introduced with hull fouling and/or shellfish imports (Photo: Björn Nadarzinski, Labor für Meeresbiologie, Werkstätten Materialhof), the marine yellow-green algae Vaucheria cf. velutina probably introduced by transoceanic shipping or with shellfish translocation (Photo: Karsten Reise), the Egyptian goose Alopochen aegyptiaca intentionally introduced by zoo and pet trade (Photo: Armin Rose), and the racoon dog Nyctereutes procyonoides is an escapee from fur-farms (Photo: Michael Gäbler/ Wikimedia Commons).
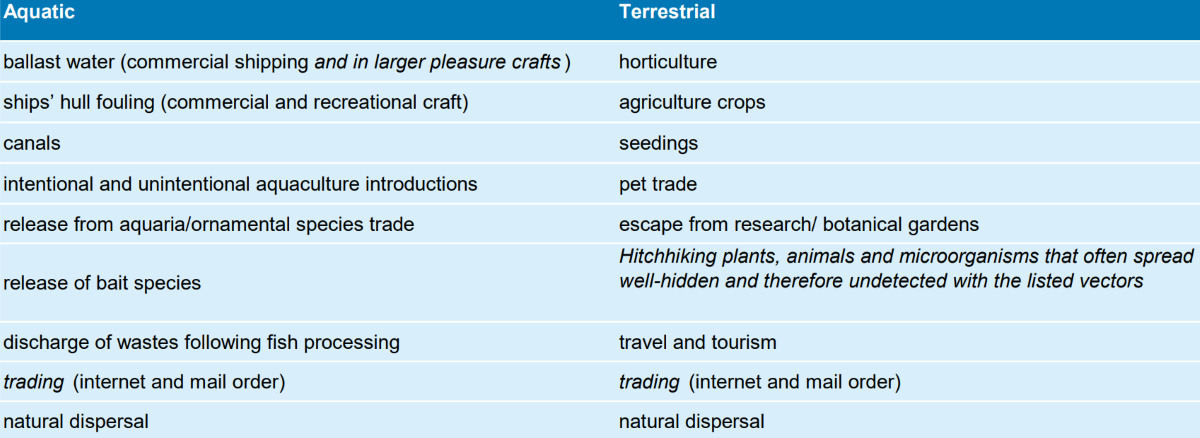 Table 1: Main possible aquatic and terrestrial vectors for global dispersal of alien species The table was taken from Schuchardt & Sevilgen (2015) who modified it after Gollasch et al. (2013) & fao.org. Additions are in italics. “Natural dispersal” as vector was added.
Table 1: Main possible aquatic and terrestrial vectors for global dispersal of alien species The table was taken from Schuchardt & Sevilgen (2015) who modified it after Gollasch et al. (2013) & fao.org. Additions are in italics. “Natural dispersal” as vector was added.
|
Definitions The definitions are cited from the given literature.
Native species (Schuchardt & Sevilgen, 2015) ”Native species" refers to a species, subspecies or genetically distinct populations, occurring within its natural range (past and present).
Alien species2,3 ”Alien species" refers to a species, subspecies or lower taxon, introduced outside its natural past or present distribution; includes any part, gametes, seeds, eggs, or propagules of such species that might survive and subsequently reproduce.
Invasive alien species2 "Invasive alien species" means an alien species whose introduction and/or spread threatens biological diversity (For the purposes of the present guiding principles, the term "invasive alien species" shall be deemed the same as "alien invasive species" in decision V/8 of the Conference of the Parties to the Convention on Biological Diversity.).
Introduction2 "Introduction" refers to the movement by human agency, indirect or direct, of an alien species outside of its natural range (past or present). This movement can be either within a country or between countries or areas beyond national jurisdiction.
Intentional introduction2 “Intentional introduction" refers to the deliberate movement and/or release by humans of an alien species outside its natural range.
Unintentional introduction2 “Unintentional introduction" refers to all other introductions which are not intentional.
Problem or nuisance species A species for which it can be assumed that based on the best available scientific evidence it will have a (significant) negative impact on the conservation goals of a Natura 2000 area.
Cryptogenic species (Tsiamis et al., 2019) “Cryptogenic species” refers to a “species with no definite evidence of their native or non-indigenous status (due to unknown origin or due to unclear mode of introduction from native range: natural spread vs human mediated)”.
Questionable species (Tsiamis et al., 2019) “Questionable species“ refers to a “NIS with insufficient information or new entries not verified by experts or NIS with unresolved taxonomic status.
Establishment "Establishment" refers to the process of an alien species in a new habitat successfully producing viable offspring with the likelihood of continued survival.
Risk analysis2 "Risk analysis" refers to: (1) the assessment of the consequences of the introduction and of the likelihood of establishment of an alien species using science-based information (i.e., risk assessment), and (2) to the identification of measures that can be implemented to reduce or manage these risks (i.e., risk management), taking into account socio-economic and cultural considerations. 1 https://www.cbd.int/decision/cop/?id=7197 |
2. Status and trends
2.1 Status and trends of marine alien species introduction in the Wadden Sea
Within the Wadden Sea Area (see WG-AS & GITTENBERGER 2019), alien species are subject to national monitoring programs. A proposal for a comprehensive trilateral monitoring was developed (Van der Have & Lensink, 2017). Previous records originate from scientific research while since 2009 various national assessments have been carried out (Gittenberger et al., 2009, 2019; Bouma et al., 2011; Schuchardt & Sevilgen, 2015; van der Have et al., 2015; Gittenberger, 2016; see also Annex) so that the knowledge on alien species in the Wadden Sea countries is continuously developing. Beyond surveys focusing on alien species detecting, several trilateral and national monitoring programs and actions, e.g. blue mussel monitoring or benthos monitoring, deliver valuable additional information about alien occurrences.
The baseline for a further trend evaluation was accomplished (Buschbaum et al., 2012; Gittenberger et al., 2015; Lackschewitz et al., 2015; Rohde et al., 2015) and these activities are relatively new as compared to all other monitoring efforts in the Wadden Sea. So far, efforts to detect alien species in the three countries mainly focused on macrofauna and -flora/macroorganisms, on (semi-)terrestrial and freshwater plants. Other groups are mostly detected by chance in the German and Danish parts of the Wadden Sea. In the Dutch part of the Wadden Sea seaweed species and gelatinous zooplankton are also specifically targeted in surveys on alien species (Gittenberger et al., 2019). Microorganisms are usually not monitored, but specific investigations provide records, such as sanitary programs on bivalve shellfish, proofed the Pacific oyster virus (Ostreid herpesvirus-1 μvar) to be present in the Wadden Sea.
The trilateral list of Wadden Sea alien species (see Annex) displays the latest status of 113 alien species recorded in the Wadden Sea Area. The list includes only marine species but ignores terrestrial and freshwater taxa occurring on the Wadden Sea islands.
The alien species inventory list of the Wadden Sea Quality Status Report 2017 included 90 species of which the ascidian Botryllus schlosseri was removed after revision because it was later considered to be native. The brackish water bivalve Rangia cuneata and the hydrozoan Garveia franciscana were only found inside a lock, but not in the Wadden Sea Area, so that the 2017 list must be reduced to 87 species in total, respectively the numbers for each country (compare Figure 2). Accordingly, 26 new marine alien species were detected until 2020 in the Wadden Sea Area.
Five species were detected in the margins (e.g., Kiel canal) of the Wadden Sea Area (Annex). Some of these alien species were included in the Trilateral alien species list of the QSR 2017. They are not included in the Trilateral alien species list of 2021 however, as it was trilaterally decided in 2019 to focus alien species management on the Wadden Sea Area, and these species were recorded outside of this area. The borders of this area are illustrated in the Trilateral Wadden Sea Management and Action Plan for Alien Species (WG-AS & Gittenberger, 2019).
In the Annex, two species, which have recently been found, are described in more detail by way of example.
2.1.1 The Netherlands
In the Netherlands, four surveys were done in 2009, 2011, 2014 and 2019, which specifically focused on the detection of alien species in the Dutch Wadden Sea (Gittenberger et al., 2009, 2010, 2012, 2015, 2019). These surveys provided almost 35 new alien species for the Dutch Wadden Sea, raising the number of registered aliens in the Dutch Wadden Sea to 90 (Gittenberger et al., 2015; Gittenberger, 2016). Many of these alien species were probably already present in the area far before the first survey in 2009. They might have been missed because the other monitoring programs focussed primarily on soft substrates and natural environments while most of the alien species in the Wadden Sea are associated with hard substrates, such as floating docks in marinas (Gittenberger et al., 2010, 2019).
2.1.2 Germany
In the German Wadden Sea, 52 alien species were registered until 2010 (Buschbaum et al., 2012). Between 2009 and 2015, 9 additional species were recorded for the first time by a rapid assessment survey (RAS) for the German Wadden Sea. Detailed information on most of the species is summarized in Lackschewitz et al. (2015). Between 2017 and 2020, another 9 species were detected, not including pelagic microbiota. Since 2009, on average about two alien species are discovered annually in the German Wadden Sea. Until 2016, 69 alien species were detected for the German Wadden Sea (Figure 2), considering also other survey data beyond the RAS (Gittenberger et al., 2013). Today, 92 alien species of the macrobiota are known for the German Wadden Sea, 103 species when the microbiota is included.
2.1.3 Denmark
For all Danish Waters (including North Sea and Skagerrak, Kattegat, Limfjorden, Belt Sea, western Baltic), 2 new species were identified per decade from 1900 to 1980, while between 1980 and 2014, 16 new species were identified per decade (Stæhr et al. 2016). The report does not provide separate data for the Danish Wadden Sea Area. Until 2016, 27 alien species were detected for the Danish Wadden Sea (Figure 2). In 2017, two new annelid species, Eteone heteropoda and Streblospio benedicti, were found at Esbjerg, raising the number of alien species to 29.
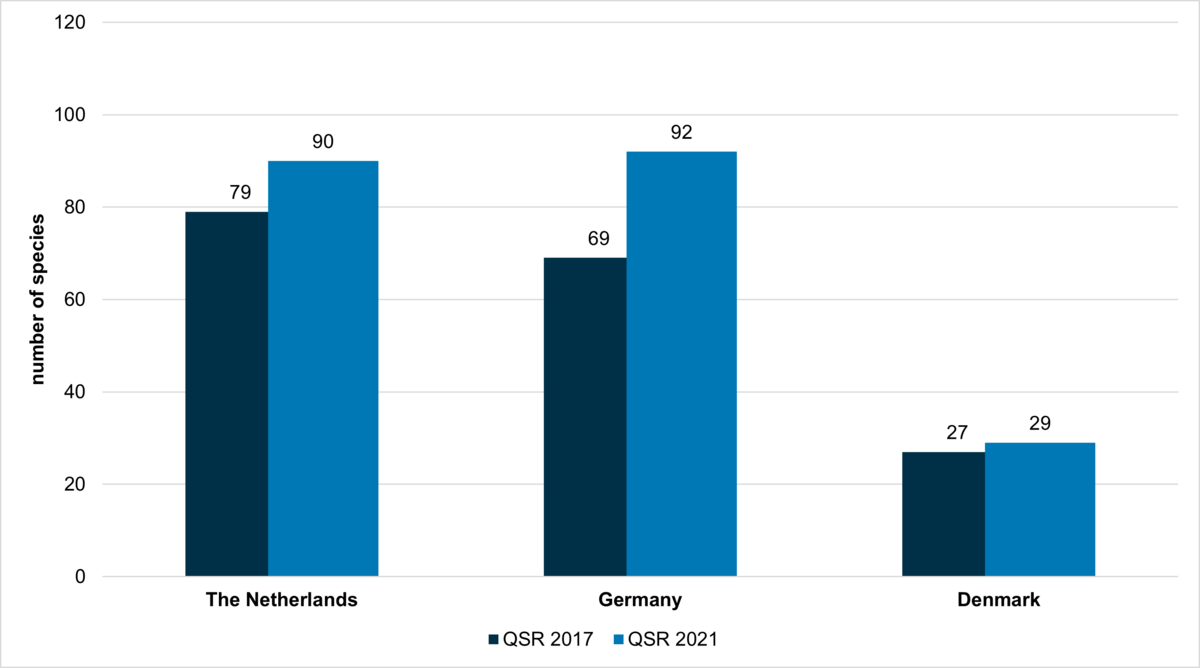 Figure 2: Number of alien benthic species ,including algae, invertebrates and one vascular plant, in the Netherlands, Germany and Denmark according to the trilateral alien species list. The figure comparatively displays the corrected data (see text) from the QSR 2017 (grey bars – Gittenberger 2016) and the updated records up to 2020 (coloured bars – Gittenberger 2021, see Annex).
Figure 2: Number of alien benthic species ,including algae, invertebrates and one vascular plant, in the Netherlands, Germany and Denmark according to the trilateral alien species list. The figure comparatively displays the corrected data (see text) from the QSR 2017 (grey bars – Gittenberger 2016) and the updated records up to 2020 (coloured bars – Gittenberger 2021, see Annex).
2.1.4 Possible vectors and species origin
Possible vectors for the introduction of marine alien species into the Wadden Sea Area (given in Figure 3) can only be identified with uncertainty because species may have been introduced by more than a single vector to various parts of the Wadden Sea. Moreover, the vectors (not including "natural distribution") responsible for the introduction into the Wadden Sea and for the initial introduction to Europe might not be identical. There are numerous examples of species that have initially been introduced to Europe by an anthropogenic vector, such as oyster imports or shipping, but subsequently spread throughout Europe by natural range expansion.
Almost half of the marine alien species originate from the Pacific, whereas almost one third of the species come from the Atlantic and few species from the Mediterranean, Ponto-Caspian or the Southern Hemisphere (Figure 4). For about 20 % of the species the origin is unknown.
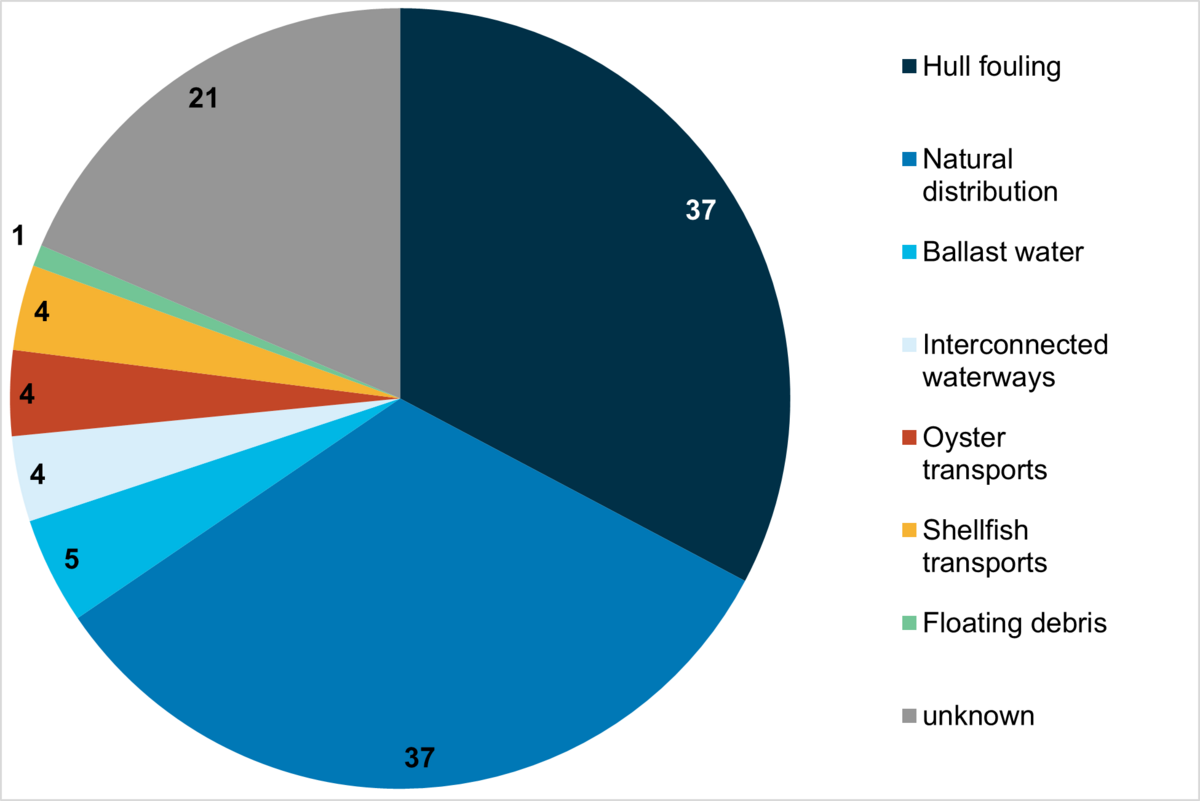 Figure 3: Possible introduction vectors of marine alien species in the Wadden Sea Area including records up to 2020 (Gittenberger 2021, see Annex). Numbers indicate the total count of taxa for each vector.
Figure 3: Possible introduction vectors of marine alien species in the Wadden Sea Area including records up to 2020 (Gittenberger 2021, see Annex). Numbers indicate the total count of taxa for each vector.
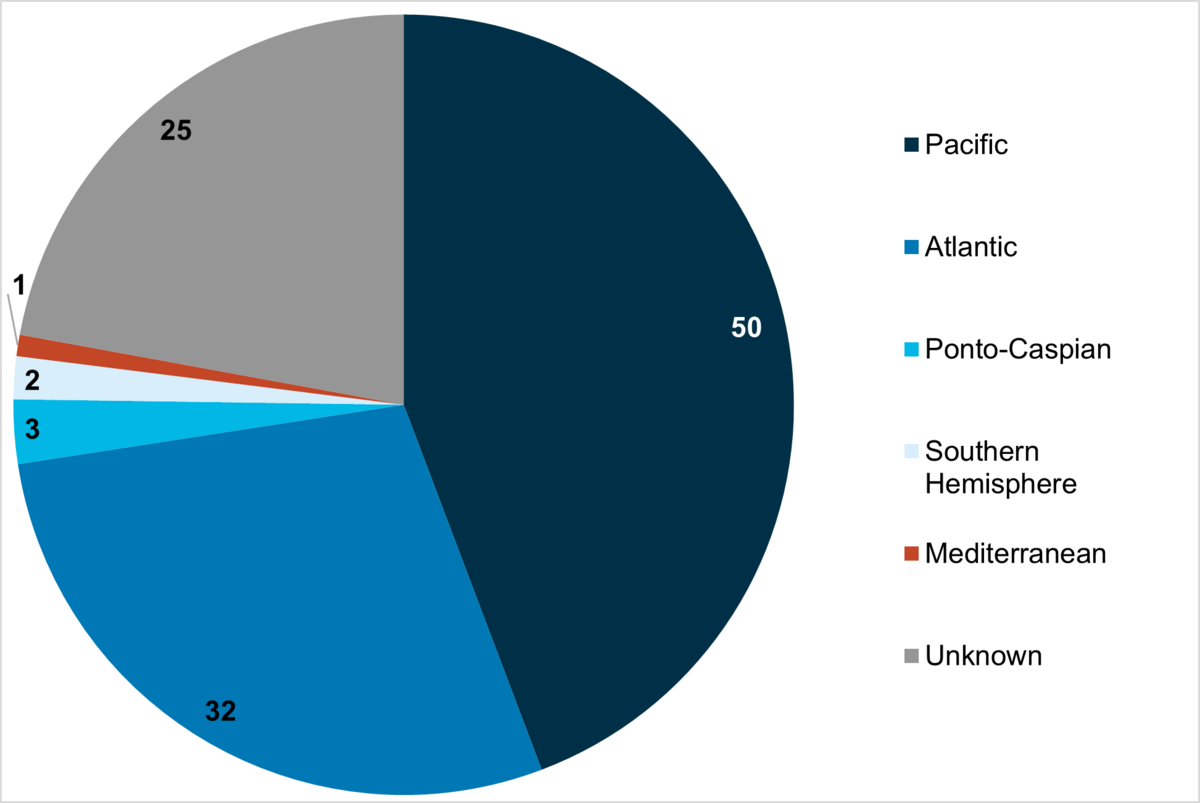 Figure 4: Origin of alien taxa in the Wadden Sea according to the trilateral alien species list (Gittenberger, 2021, see Annex). Numbers indicate the total count of taxa for each region of origin.
Figure 4: Origin of alien taxa in the Wadden Sea according to the trilateral alien species list (Gittenberger, 2021, see Annex). Numbers indicate the total count of taxa for each region of origin.
2.1.5 Introdcution rate
Between 1900 and 1989, 2-4 species were introduced per decade into the Wadden Sea Area. Since 1990 the rate increased to 10-15 species per decade (VAN DER HAVE & LENSINK 2017, Figure 5). For the period 2011-2020, the rate almost doubled, with more than 20 species added during that decade. One reason for the high number of alien species recorded since ~2009 is the increased monitoring effort and awareness of alien species. Before that time, no targeted surveys throughout the Wadden Sea have been conducted with a specific focus on the detection of alien species.
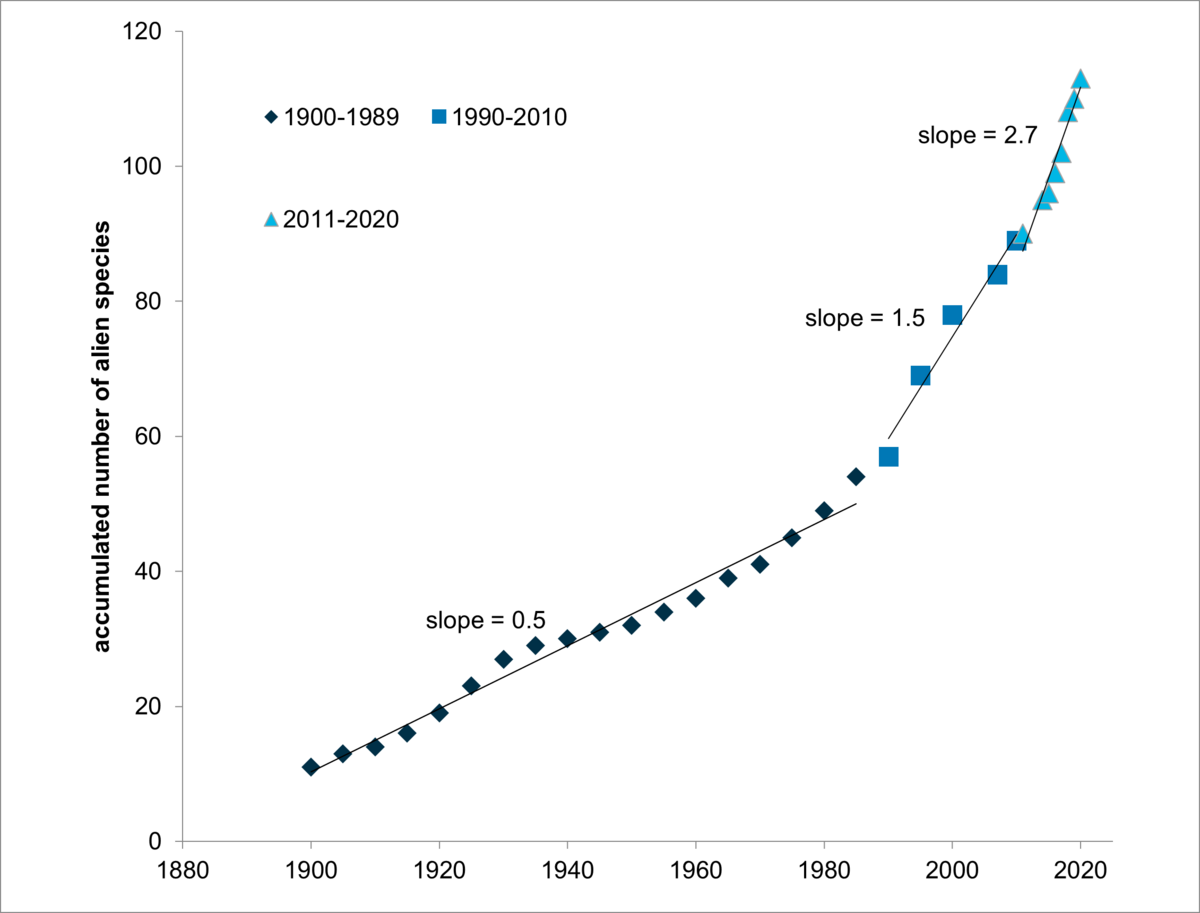 Figure 5: The accumulated number of alien species in the trilateral Wadden Sea Area is presented for the periods 1900-1989, 1990-2010 and 2011-2020. For each species, the year of the first proof of introduction was used (Gittenberger, 2021). The time periods are chosen for comparison with the cumulative number of alien species in Danish marine surveys (Stæhr et al., 2016). Figure taken from Van der Have & Lensink (2017) and updated.
Figure 5: The accumulated number of alien species in the trilateral Wadden Sea Area is presented for the periods 1900-1989, 1990-2010 and 2011-2020. For each species, the year of the first proof of introduction was used (Gittenberger, 2021). The time periods are chosen for comparison with the cumulative number of alien species in Danish marine surveys (Stæhr et al., 2016). Figure taken from Van der Have & Lensink (2017) and updated.
2.2 Status and trends of terrestrial and freshwater alien species introduction in the Wadden Sea Area
Alien species occur in all terrestrial habitats in the Wadden Sea region, from intertidal salt marshes via dunes and dune slacks to forests, and in freshwater habitats like ponds and streams. There is no trilaterally harmonized monitoring or management program on alien species in the terrestrial realm of the Wadden Sea Cooperation Area. Moreover, reliable data for assessing the overall changes in the number and abundance of alien species or their effects on biodiversity and functioning of ecosystems are lacking.
Lensink et al. (2015) provided a list of (potentially) threatening alien species on the Dutch Wadden Sea islands and a chapter on terrestrial alien species in the German Wadden Sea is included in Schuchardt & Sevilgen (2015). A first overview of alien species on the German and Danish islands was prepared (Büttger et al., 2017), but a common list for the entire Wadden Sea region is still missing.
2.2.1 Freshwater and terrestrial vascular plants
In 2016, the European Union published a first list of Invasive Alien Species of Union concern, which was updated in 2017 and 2019 (https://ec.europa.eu/environment/nature/invasivealien/list/index_en.htm). Currently, this list contains 62 freshwater and terrestrial vascular plants, of which 20 occur in at least one of the countries of the Trilateral Wadden Sea cooperation. Baccharis halimifolia (Eastern baccharis) is the only plant of these 20 species, which is directly associated with characteristic Wadden Sea habitats (dunes and salt marshes). In addition, 13 of the 20 plants of ‘Union concern’ occurring in Denmark, Germany and/or the Netherlands occur in freshwater ecosystems like wetlands, ponds, lakes or streams, and could thus potentially occur also in the Wadden Sea region.
According to lists on alien species of the Wadden Sea in The Netherlands and Germany provided by Lensink et al. (2015) and Schuchardt & Sevilgen (2015), a large share belongs to vascular plants (e.g. Spartina anglica; Rosa rugosa, Figure 6, Figure 7), but also mosses (Campylopus introflexus) and animals of various taxonomic groups (Branta canadensis, Dreissenia polymorpha, Cyprinus carpio, Felis catus) are considered invasive aliens.
Many plant species, nowadays considered as invasive aliens in the Wadden Sea region, were intentionally introduced by humans because of their ‘beneficial’ effects: Spartina anglica on tidal flats for coastal erosion control, Rosa rugosa to decrease sand drift in dunes and as a beautiful ornamental plant, Prunus serotina for improving the nutrient availability in nutrient poor coastal forests. Furthermore, aliens threatening the species-rich vegetation of dune slacks like Crassula helmsii are widely reared in aquaria or garden ponds and have escaped or been released from these anthropogenic habitats. Overall, there is a clear positive relation between the number of human inhabitants per island and the occurrence of aliens on Dutch Wadden Sea islands (Lensink et al., 2015). The distance to roads or houses is a major predictor for the occurrence of Rosa rugosa in dunes in Denmark (Kollmann et al., 2009).
 Figure 6: Common Cordgrass (Spartina anglica) in the Wadden Sea. Detailed picture (right) and overview of a local Spartina-population (left) (Photos: Kai Jensen).
Figure 6: Common Cordgrass (Spartina anglica) in the Wadden Sea. Detailed picture (right) and overview of a local Spartina-population (left) (Photos: Kai Jensen).
 Figure 7: Rugosa rose (Rosa rugosa) (Photo: Martin Stock).
Figure 7: Rugosa rose (Rosa rugosa) (Photo: Martin Stock).
2.2.2 Vertebrates
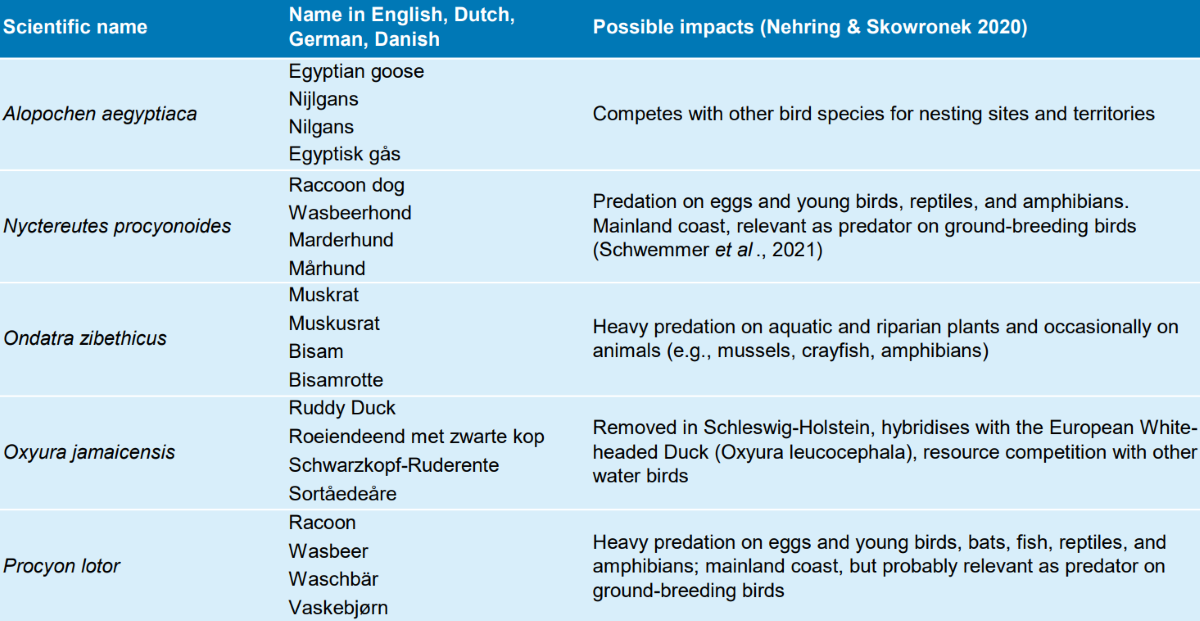
Several alien vertebrate species occur in the Wadden Sea region. Of these, some are listed on the Union list (see also 2.2.1. and Table 2). Various species, e.g. racoon dog, live on the mainland coast but have a relevant impact as predator on ground-breeding birds (Schwemmer et al., 2021).
Table 2: Examples of wild living alien vertebrates of the Union list in the Wadden Sea region and their possible impacts (Lensink et al. 2015; The Danish Environmental Protection Agency 2017; Nehring & Skowronek 2020).
2.2.3 Alien to the area or not?
It is often challenging to determine whether a species is to be considered native or alien. While foxes are native to the Wadden Sea region, they have not been established naturally on the Wadden Sea islands and thus they could be considered alien species there. Another example is the recent invasion of dunes in Denmark by the grass Deschampsia flexuosa, which is native and wide-spread in inland ecosystems but has been absent from coastal dunes half a century ago (Nielsen et al., 2011). Finally, it may be questioned whether the grass Spartina anglica is an alien in the Wadden Sea region at all. The species was considered the ‘most important invasive plant in the Wadden Sea’ by Nehring et al., (2009), and it is listed by the World Conservation Union to be among the 100 ‘World’s Worst’ invaders (Lowe et al., 2000). Spartina anglica ‘naturally evolved’ by spontaneous hybridization between a native European and an introduced American Spartina species and subsequent ‘natural polyploidization’ in southern England by the end of the 19th century. Later, the species was deliberately introduced and planted in the Wadden Sea region but would probably also have expanded naturally from Southern England to the Wadden Sea (Annex and Thematic Report on Salt Marshes).
Although S. anglica was extensively used as the example of a species with great negative impact on native biodiversity and ecosystem functioning, it is not listed as ‘invasive alien species of union concern’ (EU 2016, 2017, 2019). Recently, Granse et al. (2021) indeed documented positive effects of the invasion of Spartina anglica on both native plant species richness and on accretion rates in Wadden Sea salt marshes. In addition, LANGE et al. (2019) showed in a mesocosm experiment, that marine consumer species differ in the use of Spartina anglica as food source. The native surface deposit feeding snail Peringia ulvae was able to increase its biomass using S. anglica as food source.
3. Assessment
In accordance with the UNESCO World Heritage Committee request of 2009, §26 of the Sylt declaration (2010) and §33 of the Tønder Declaration (2014), the Wadden Sea Board initiated the development of a common strategy for dealing with alien species in the Wadden Sea (see Annex) and the TWSC`s ad hoc Expert Group Alien Species (EG-AS) developed the trilateral alien species management and action plan (MAPAS ) (WG-AS & Gittenberger, 2019, see also Annex), in order “to prevent threats from alien species to marine and terrestrial ecosystems in the Wadden Sea Area and to sustain the Outstanding Universal Value (OUV) and integrity of the Wadden Sea World Heritage property by preventing, managing or controlling alien species through a coordinated effort, in line with international conventions and treaties, the EU directive on invasive alien species (EU-IAS), the Marine Strategy Framework Directive (MSFD) and other relevant policies”.
The following assessment of this thematic report focuses on the effects of alien species on the Wadden Sea Plan targets (chapter 3.1) and the criteria for the ‘Outstanding Universal Value’ (OUV; chapter 3.2).
So far, alien species have not caused the extinction of native species in the Wadden Sea and most alien species become inconspicuous residents. However, some alien species have the potential to alter dominance structures, habitats, and trophic regimes, which are fundamental for the ecosystem and to the guiding principle of the trilateral Wadden Sea policy, the Wadden Sea Plan targets as well as for ‘Outstanding Universal Value’ (OUV). It clarifies the need to implement the MAPAS.
3.1 Wadden Sea Plan Targets
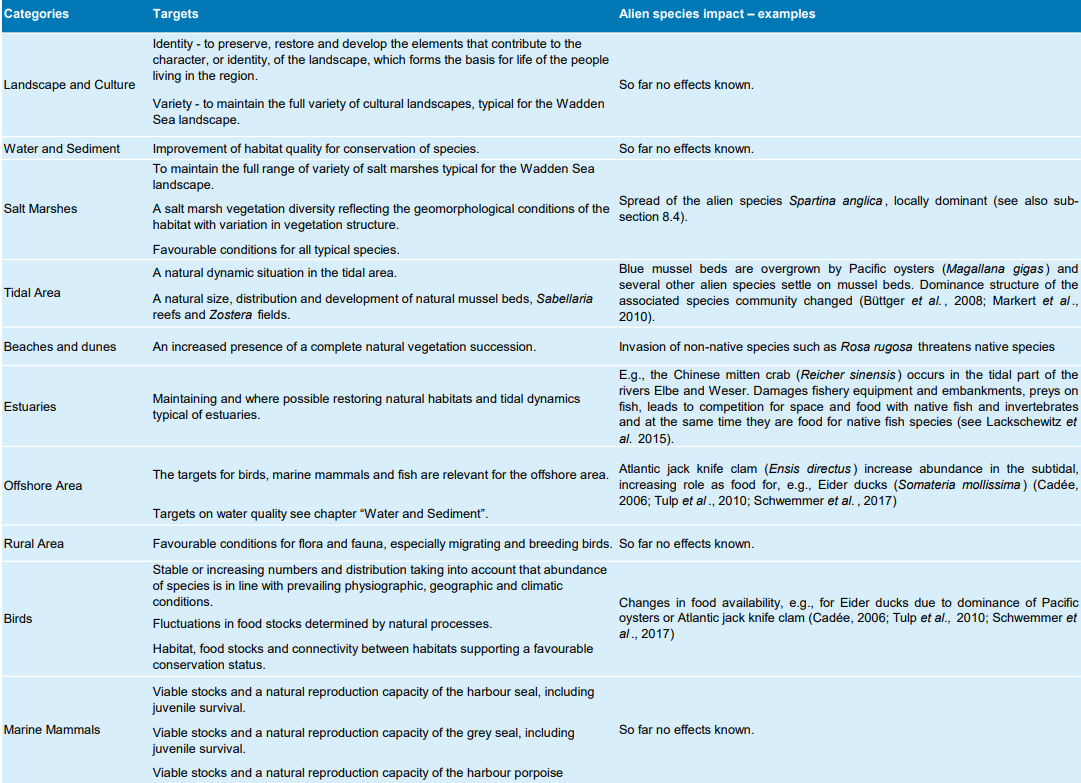
In the Wadden Sea Plan 2010 (CWSS, 2010b) several habitat targets were defined, which might be affected by alien species, but no specific targets for alien species were formulated. Table 3 summarizes those targets and lists examples of the effects of aliens and measures taken.
3.2 Outstanding Universal Value (OUV)
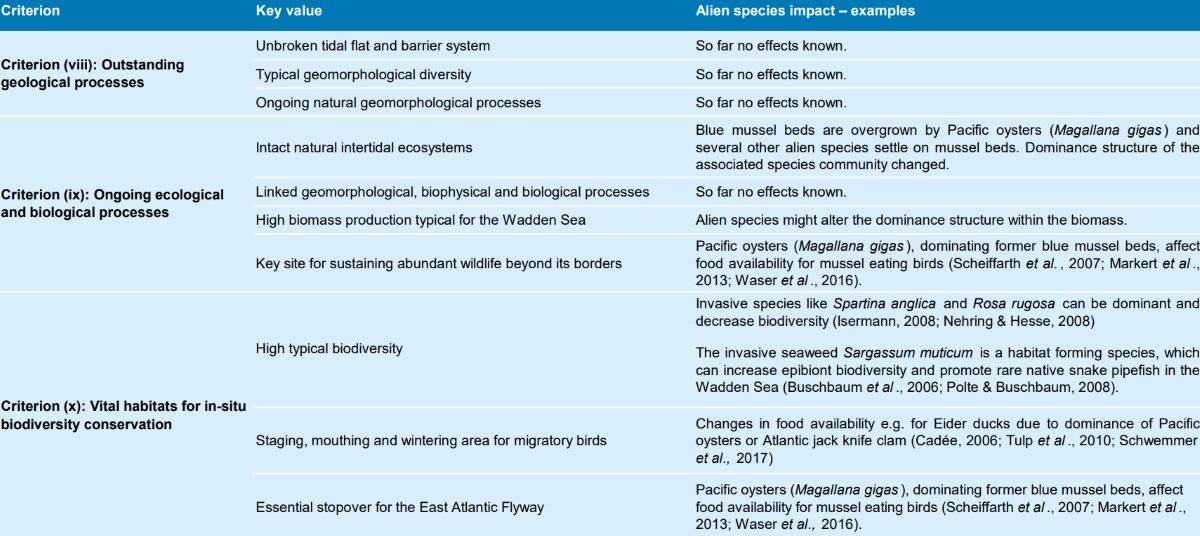
The Wadden Sea was inscribed on the World Heritage List under the natural criteria (viii), (ix) and (x) for the ‘Outstanding Universal Value’ (OUV). Table 4 gives an overview of these criteria and their key values and how they might be affected by alien species. With the inscription of the Wadden Sea on the World Heritage List, the World Heritage Committee recommended to “implement a monitoring programme on invasive species associated with ballast water and aquaculture”.
Table 3: Targets of the Wadden Sea Plan 2010 (CWSS, 2010b), which might be affected by alien species. Examples of alien species impacts are given.
Table 4: Key values of the OUV, which might be affected by alien species.
4. Recommendations
Following the status and increasing trend of alien species introduction and the trilateral progress in alien species assessment in the Wadden Sea Area, recommendations for monitoring, management and research are given.
4.1 Science
-
Effects of alien species on food webs and communities need higher attention in research.
-
Knowledge about the effects of non-native parasites and their interactions with native species is still scarce and should be given more attention.
-
The potential benefits (early detection, easy and efficient) and disadvantages of the eDNA and other DNA-based methodologies to detect alien species should be further investigated. This study can be based on several recent (pilot) studies done in the Danish, German and Dutch parts of the Wadden Sea (Markert et al., 2014; Gittenberger et al., 2019; Andersen et al., in prep., Project "Genetic tools for Ecosystem health Assessment in the North Sea region" (GEANS)).
4.2 Monitoring
-
Fully operationalize the proposed harmonized monitoring program in the MAPAS (Management and action plan for Alien Species) for alien species in the Wadden Sea. The monitoring proposal is mostly in line with the requirements of OSPAR and MSFD (WG-AS & Gittenberger, 2019).
-
So far, detection of alien species in the Wadden Sea is focussing on macrobenthic species in harbours, marinas and at shellfish farming sites as well as on terrestrial and freshwater species. Future monitoring should also consider phytoplankton, zooplankton, fish, and birds. The observation of viruses and other microorganisms should not be disregarded completely, although knowledge about these taxonomic groups is still very limited and should be considered for future research, to create a baseline.
-
Different substrates for settling/ habitats need to be monitored (tidal flats and subtidal soft bottom well as artificial hard substrates). The monitoring of alien species in salt marshes and dunes could be easily incorporated in the existing monitoring procedures. The monitoring program needs a solid financial fundament and has to be in line with the requirements of the MSFD for Descriptor 2 and Regulation 1143/2014 on invasive alien species.
-
The trilateral alien species list should cover zooplankton, phytoplankton, fish, birds and terrestrial taxa as well.
-
A common trilateral portal should be established to support networking and to exchange knowledge about the Wadden Sea alien species list as well as species alerts.
4.3 Management
-
A common and continuously updated trilateral list of aquatic and terrestrial alien species based on national and trilateral monitoring programs is needed.
-
MAPAS and the Sylt Declaration refer to the Wadden Sea Area for alien species: e.g., marine aliens species found inside locks would not belong to the species list although they might reach the Wadden Sea Area easily. These should be included in the list, marked accordingly,
-
A trilateral Wadden Sea early warning and reporting system is needed. Supporting elements for such a system could include a network platform, which will be a microsite attached to the website of the CWSS or a harmonised guideline for risk assessment, which will be integrated into the website of the CWSS. This platform could also provide a harmonised guideline for the risk assessment of newly established non-native species
-
Already established populations of alien species cannot be removed. All measures for managing such alien species should be based on a critical evaluation of collateral damages.
-
Eradication has the risk of unforeseeable collateral damages. Therefore, introduction of alien species should be prevented by limiting the chances of introduction through the vectors.
-
The operationalization of the Wadden Sea Area communication and education plan for alien species needs to be developed and supported.
-
Next to ballast water transport, ship biofouling is the most important vector for the introduction of alien species into the Wadden Sea and adjacent areas. Therefore, a harmonized approach to define options for the implementation of the IMO's Guidance (recreational boats) and Guidelines for the Control and Management of Ships' Biofouling is needed.
-
Aquaculture related import and transport activities may support the spread of alien species if left unmanaged. A trilateral common policy on potential shipping and shellfish transportation related transport vectors and pathways of alien species into and within the Wadden Sea, may be developed using the Trilateral Wadden Sea Management and Action Plan for Alien Species (WG-AS & Gittenberger, 2019) as a baseline. In addition to human mediated spread and establishment of NIS, natural dispersal capacities and habitat preferences should be considered.
5. Summary
So far, there is no evidence that alien species have caused the extinction of native species in the Wadden Sea, and most alien species become inconspicuous residents. However, some alien species have the potential to alter dominance structures, habitats, and trophic regimes.
In accordance with the UNESCO World Heritage Committee request of 2009, the Sylt declaration (2010) and the Tønder Declaration (2014), the Wadden Sea Board initiated the development of a common strategy for dealing with alien species in the Wadden Sea and the TWSC`s ad hoc Expert Group Alien Species (EG-AS) developed the trilateral alien species management and action plan (MAPAS) (WG-AS & Gittenberger, 2019).
Currently, the trilateral alien species list of the Wadden Sea Area focusses on marine species and comprises 113 species, not including birds, invertebrates and other terrestrial species. Between 1900 and 1989, 2-4 species were introduced per decade into the Wadden Sea Area. Since 1990 the rate increased to 10-15 species per decade (Van der Have & Lensink, 2017). For the period 2011-2020, the rate almost doubled, with more than 20 species added during that decade. Most of these marine alien taxa originate from the Pacific or from the Atlantic. Major vectors are transoceanic shipping and aquaculture; intentional or unintentional introduction (horticulture, pet trade and transport) is the most important pathway for terrestrial plants. A common trilateral alien species list for terrestrial taxa of the Wadden Sea Area is still missing.
The following assessment of this thematic report focuses on the effects of alien species on the Wadden Sea Plan targets (chapter 3.1) and the criteria for the ‘Outstanding Universal Value’ (OUV; chapter 3.2).
In summary, there is no evidence that alien species endanger the Wadden Sea Plan targets and the natural criteria (viii), (ix) and (x) for the ‘Outstanding Universal Value’ (OUV) fundamentally. They have not caused the extinction of native species in the Wadden Sea so far, and most alien species become inconspicuous residents. However, some alien species have the potential to alter dominance structures, habitats, and trophic regimes, which are fundamental for the ecosystem and the Wadden Sea Plan targets as well as for ‘Outstanding Universal Value’ (OUV). It clarifies the need to implement the MAPAS.
The given recommendations focus on the implementation of a harmonized alien species monitoring program and a continuously updated trilateral list including macrobenthos, algae, phytoplankton, zooplankton, fish, birds and terrestrial species (e.g., plants, mammals).
About the authorsBüttger, H.1, Christoph, S.1, Buschbaum, C.2, Gittenberger, A.3, Jensen, K.4, Kabuta, S.5, Lackschewitz, D.2
1 BioConsult SH GmbH & Co. KG, Schobüller Str. 36, 25813 Husum, DE 2 AWI Sylt, Hafenstraße 43, 25992 List/Sylt, DE 3 GiMaRIS, Rijksstraatweg 75, 2171 AK Sassenheim, NL 4 University of Hamburg, Ohnhorststr. 18, 22609 Hamburg, DE 5 Rijkswaterstaat, Ministry of Infrastructure and the Environment, Amsterdam, NL |
|
Aknowledgements We thank the reviewers for their valuable comments which have improved this report considerably. We also thank Jesper Andersen (NIVA Denmark) who provided us with up-to-date information on alien species in the Danish Wadden Sea. And, of course, thanks go to all those who are monitoring in the Wadden Sea, on the islands and Halligen, in the saltmarshes and in the dunes, keeping their eyes and ears open and recording and reporting new non-native species. |
References
Andersen, J. H., E. Kallenbach, M. B. Kieldgaard, S. W. Knudsen, T. Dale, W. Eikrem, C. Fagerli, G. Green, A. Hobæk, E. Oug, J. Thaulow, M. Hesselsøe, D. Bekkevold, L. M. W. Jacobsen, J. Kuhn, P. R. Møller, C. A. Olesen, H. Carl & F. Stuer-Lauridsen. in prep. A baseline study of the occurrence of non-indigenous species in Danish harbours.
Andersen, J. H., S. A. Pedersen, J. Thaulow, F. Stuer-Lauridsen, D. Kristensen & S. Cochrane. 2014. Monitoring of non-indigenous species in Danish marine waters - Background and proposals for a monitoring strategy and a monitoring network. Copenhagen (DNK): 55
Bouma, S., S. Gollasch & W. Lengkeek. 2011. Neobiota in the Wadden Sea. Including recommendations for a trilateral strategy. Wilhelmshaven (DEU): 80
Buschbaum, C., A. S. Chapman & B. Saier. 2006. How an introduced seaweed can affect epibiota diversity in different coastal systems. Marine Biology 148/4: 743–754.
Buschbaum, C., D. Lackschewitz & K. Reise. 2012. Nonnative macrobenthos in the Wadden Sea ecosystem. Ocean & Coastal Management 68: 89–101.
Büttger, H., S. Alpert, T. M. Van der Have & C. Goldberg. 2017. Alien species on the German and Danish islands. 51
Büttger, H., H. Asmus, R. Asmus, C. Buschbaum, S. Dittmann & G. Nehls. 2008. Community dynamics of intertidal soft-bottom mussel beds over two decades. Helgoland Marine Research 62/1: 23–36.
Cadée, G. C. 2006. Eidereenden op Ensis directus dieet. Spirula 351: 86–87.
Capinha, C., F. Essl, H. Seebens, D. Moser & H. M. Pereira. 2015. The dispersal of alien species redefines biogeography in the Anthropocene. Science 348/6240: 1248–1251.
Common Wadden Sea Secretariat - CWSS. 2010a. Sylt Declaration. Ministerial Council Declaration of the Eleventh Trilateral Governmental Conference on the Protection of the Wadden Sea. (Common Wadden Sea Secretariat). Wilhelmshaven (DEU)
Common Wadden Sea Secretariat - CWSS. 2010b. Wadden Sea Plan 2010. conf.: 11th Trilateral Governmental Conference on the Protection of the Wadden Sea, Westerland/Sylt 18 March 2010. Wilhelmshaven (DEU).
Common Wadden Sea Secretariat - CWSS. 2014. Toender Declaration. Ministerial Council Declaration of the 12th Trilateral Governmental Conference on the Protection of the Wadden Se. (Common Wadden Sea Secretariat). Wilhelmshaven, Germany
Faasse, M., L. Verboom & L. van der Loos. 2018. De Pacifische vlokreeft Aoroides semicurvatus in Nederland. Macrofaunanieuwsmail. Helpdesk Water, Rijkswaterstaat WVL: Utrecht 140: 21–23.
Gittenberger, A. 2016. Alien species of the Wadden Sea database.
Gittenberger, A. 2021. Alien species of the Wadden Sea database.
Gittenberger, A., M. Rensing, R. Dekker, P. Niemantsverdriet, N. Schrieken & H. Stegenga. 2015. Native and non-native species of the Dutch Wadden Sea in 2014. Leiden & Den Burg (NLD): 94
Gittenberger, A., M. Rensing, P. Niemantsverdriet, N. Schrieken & H. Stegenga. 2013. Native and non-native species of hard substrata in the Schleswig-Holstein Wadden Sea 2012-2013. 105
Gittenberger, A., M. Rensing, N. Schrieken & H. Stegenga. 2012. Waddenzee inventarisatie van aan hard substraat gerelateerde organismen met de focus op exoten, zomer 2011. i.o.v. de Producentenorganisatie van de Nederlandse Mosselcultuur: 61
Gittenberger, A., M. Rensing, H. Stegenga & B. W. Hoeksema. 2009. Inventarisatie van de aan hard substraat gerelateerde macroflora en macrofauna in de Nederlandse Waddenzee. Leiden (NLD): 63
Gittenberger, A., M. Rensing, H. Stegenga & B. Hoeksema. 2010. Native and non-native species of hard substrata in the Dutch Wadden Sea. Nederlandse Faunistische Mededelingen 33: 21–75.
Gittenberger, A., M. Rensing, T. M. Van der Have, C. J. M. Philippart, van der Hoorn, B., D’Hont, A., Wesdorp, K. H., Schrieken, N., Klunder, L., Kleine-Schaars, L., S. Holthuijsen & H. Stegenga. 2019. Native and non-native species of the Dutch Wadden Sea in 2018. 128
Gittenberger, A., K. H. Wesdorp & M. Rensing. 2017. Biofouling as a transport vector of non-native marine species in the Dutch Delta, along the North Sea coast and in the Wadden Sea. Leiden (NLD)
Gollasch, S., D. Minchin, B. Galil, A. Occhipinti-Ambrogi, A. Marchini & S. Olenin. 2013. VECTORS fact sheet series, Invasive Alien Species.
Gouillieux, B., N. Lavesque, J.-C. Leclerc, V. Le Garrec, F. Viard & G. Bachelet. 2016. Three non-indigenous species of Aoroides (Crustacea: Amphipoda: Aoridae) from the French Atlantic coast. Journal of the Marine Biological Association of the United Kingdom 96/8: 1651–1659. DOI: 10.1017/S0025315415002027, ISSN: 0025-3154, 1469-7769.
Granse, D., S. Suchrow & K. Jensen. 2021. Long-term invasion dynamics of Spartina increase vegetation diversity and geomorphological resistance of salt marshes against sea level rise. Biological Invasions 23/3: 871–883. DOI: 10.1007/s10530-020-02408-0, ISSN: 1387-3547, 1573-1464.
van der Have, T. M., B. van den Boogard, R. Lensink, D. Poszig & C. J. M. Philippart. 2015. Alien species in the Dutch Wadden Sea: policies and management. Culemborg (NDL) & Den Burg (NDL): 123
Isermann, M. 2008. Expansion of Rosa rugosa and Hippophaë rhamnoides in coastal grey dunes. Flora - Morphology Distribution Functional Ecology of Plants 203/4: 273–280.
Kollmann, J., R. H. Jørgensen, J. Roelsgaard & H. Skov-Petersen. 2009. Establishment and clonal spread of the alien shrub Rosa rugosa in coastal dunes—A method for reconstructing and predicting invasion patterns. Landscape and Urban Planning 93/3–4: 194–200. DOI: 10.1016/j.landurbplan.2009.07.006, ISSN: 01692046.
Kornis, M. S., N. Mercado-Silva & J. Vander Zanden. 2012. Twenty years of invasion: a review of round goby Neogobius melanostomus biology, spread and ecological implications. Journal of Fish Biology 80/2: 235–285.
Krieg, H., T. Eller & L. Kies. 1988. Verbreitung und Ökologie der Vaucheria-Arten (tribophyceae) des Elbe-Ästuars und der angrenzenden Küste. Helgoländer Meeresuntersuchungen 42: 613–636.
eds. Lackschewitz, D., K. Reise, C. Buschbaum, R. Karez, Landesamt für Landwirtschaft, Umwelt und Ländliche Räume des Landes Schleswig-Holstein, & Alfred-Wegener-Institut. 2015. Neobiota in deutschen Küstengewässern: eingeschleppte und kryptogene Tier- und Pflanzenarten an der deutschen Nord- und Ostseeküste. (Datenstand: Juli 2014. edition). collection: LLUR SH Gewässer no. D 25, LLUR/Flintbek, 216. ISBN: 978-3-937937-73-1.
Lange, G., J. A. Schmitt, I. Kröncke, S. D. Moorthi, S. Rohde, S. Scheu & P. J. Schupp. 2019. The role of invasive marine plants for macrofauna nutrition in the Wadden Sea. Journal of Experimental Marine Biology and Ecology 512: 1–11. DOI: 10.1016/j.jembe.2018.12.005, ISSN: 00220981.
Lensink, R., T. M. van de Have, R. J. van der Haterd, J. A. Inberg, D. M. Soes & B. Achterkamp. 2015. Alien species on the Dutch Wadden Sea Islands Occurrence and ecological risks. Culemborg (NDL): 110
Lieberum, C. & G. Bock. 2016. Neobiota Standard.
Lowe, S., M. Browne, S. Boudjelas & M. De Poorter. 2000.100 of the World’s Worst Invasive Alien Species, A selection from the Global Invasive Species. Database.12
Markert, A., W. Esser, D. Frank, A. Wehrmann & K.-M. Exo. 2013. Habitat change by the formation of alien Crassostrea-reefs in the Wadden Sea and its role as feeding sites for waterbirds. Estuarine, Coastal and Shelf Science 131: 41–51.
Markert, A., M. J. Raupach, A. Segelken-Voigt & A. Wehrmann. 2014. Molecular identification and morphological characteristics of native and invasive Asian brush-clawed crabs (Crustacea: Brachyura) from Japanese and German coasts: Hemigrapsus penicillatus (De Haan, 1835) versus Hemigrapsus takanoi Asakura & Watanabe 2005. Organisms Diversity & Evolution 14/4: 369–382. DOI: 10.1007/s13127-014-0176-4, ISSN: 1439-6092, 1618-1077.
Markert, A., A. Wehrmann & I. Kröncke. 2010. Recently established Crassostrea-reefs versus native Mytilus-beds: differences in ecosystem engineering affects the macrofaunal communities (Wadden Sea of Lower Saxony, southern German Bight). Biological Invasions 12/1: 15–32. DOI: 10.1007/s10530-009-9425-4, ISSN: 1387-3547, 1573-1464.
McGrannachan, C. M., S. Pagad & M. A. McGeoch. 2021. A multiregional assessment of transnational pathways of introduction. NeoBiota 64: 43–67. DOI: 10.3897/neobiota.64.60642, ISSN: 1314-2488, 1619-0033.
Leppäkoski, E., S. Gollasch & S. Olenin. 2002. Biological invasions into German waters: An evaluation of the importance of different human-mediated vectors for nonindigenous macrozoobenthic species. in: Invasive Aquatic Species of Europe: Distributions, Impacts and Management. Kluwer Academic Publishers/Dordrecht (NLD), 373–383.
Nehring, S. & K.-J. Hesse. 2008. Invasive alien plants in marine protected areas: the Spartina anglica affair in the European Wadden Sea. Biological Invasions 10: 937–950.
Nehring, S., K. Reise, N. Dankers & P. S. Kristensen. 2009. Alien Species. Thematic Report No. 7. in: Quality Status Report 2009 (from: Marencic, H. & J. De Vlas). collection: Wadden Sea Ecosystem No. 25, Common Wadden Sea Secretariat, Trilateral Monitoring and Assessment Group/Wilhelmshaven (DEU), 28.
Nehring, S. & S. Skowronek. 2020. Die invasiven gebietsfremden Arten der Unionsliste der Verordnung (EU) Nr. 1143/2014 – Zweite Fortschreibung 2019 –. Bonn - Bad Godesberg (DEU): 191
Nielsen, K. E., H. J. Degn, C. Damgaard, M. Bruus & B. Nygaard. 2011. A Native Species with Invasive Behaviour in Coastal Dunes: Evidence for Progressing Decay and Homogenization of Habitat Types. AMBIO 40/7: 819–823. DOI: 10.1007/s13280-011-0144-6, ISSN: 0044-7447, 1654-7209.
Polte, P. & C. Buschbaum. 2008. Native pipefish Entelurus aequoreus are promoted by the introduced seaweed Sargassum muticum in the northern Wadden Sea, North Sea. Aquatic Biology 3: 11–18.
Reise, K., N. Dankers & K. Essink. 2005. Introduced Species. Wadden Sea Ecosystem No. 19 155–161.
Riemann, B., J. Carstensen, K. Dahl, H. Fossing, J. W. Hansen, H. H. Jakobsen, A. B. Josefson, D. Krause-Jensen, S. Markager, P. A. Stæhr, K. Timmermann, J. Windolf & J. H. Andersen. 2016. Recovery of Danish Coastal Ecosystems After Reductions in Nutrient Loading: A Holistic Ecosystem Approach. Estuaries and Coasts 39/1: 82–97. DOI: 10.1007/s12237-015-9980-0, ISSN: 1559-2723, 1559-2731.
Rohde, S., A. Markert, P. Schupp & A. Wehrmann. 2015. Neobiota-Basislinie in niedersächsischen Küstengewässern. Wilhelmshaven (DEU), Bericht erstellt im Auftrag des NLWKN und NLPV: 72
Scheiffarth, G., B. Ens & A. Schmidt. 2007. What will happen to birds when Pacific Oysters take over the mussel beds in the Wadden Sea? Wadden Sea Newsletter 1: 10–14.
BioConsult Schuchard & Scholle (ed.). 2015. Invasive alien species (IAS) policies and management in the German Wadden Sea. (BioConsult Schuchard & Scholle). Bremen(DEU), im Auftrag des Common Wadden Sea Secretariat (CWSS): 71
Schwemmer, P., K. Eskildsen, L. Enners, S. Horn, K. Wittbrodt, M. Stage, K. Binder, H. Büttger, A. Ruales, K. Stelzer, H. Asmus, R. Asmus, S. Garthe, J. Kohlus, H.-C. Reimers, K. Ricklefs & K. Schwarzer. 2017. STopP ‘Vom Sediment zum Top-Prädator’. Tönning (DEU), Partner: AWI-Wattenmeeerstation, FTZ-Westküste, IfG der CAU, LLUR, LKN-NPV: 90
Schwemmer, P., S. Weiel & S. Garthe. 2021. Spatio-temporal movement patterns and habitat choice of red foxes (Vulpes vulpes) and racoon dogs (Nyctereutes procyonoides) along the Wadden Sea coast. European Journal of Wildlife Research 67/3: 49. DOI: 10.1007/s10344-021-01474-6, ISSN: 1612-4642, 1439-0574.
Seebens, H., T. M. Blackburn, E. E. Dyer, P. Genovesi, P. E. Hulme, J. M. Jeschke, S. Pagad, P. Pyšek, M. Winter, M. Arianoutsou, S. Bacher, B. Blasius, G. Brundu, C. Capinha, L. Celesti-Grapow, W. Dawson, S. Dullinger, N. Fuentes, H. Jäger, J. Kartesz, M. Kenis, H. Kreft, I. Kühn, B. Lenzner, A. Liebhold, A. Mosena, D. Moser, M. Nishino, D. Pearman, J. Pergl, W. Rabitsch, J. Rojas-Sandoval, A. Roques, S. Rorke, S. Rossinelli, H. E. Roy, R. Scalera, S. Schindler, K. Štajerová, B. Tokarska-Guzik, M. van Kleunen, K. Walker, P. Weigelt, T. Yamanaka & F. Essl. 2017. No saturation in the accumulation of alien species worldwide. Nature Communications 8/1: 14435.
Stæhr, P. A., H. H. Jakobsen, J. L. S. Hansen, P. Andersen, M. Storr-Paulsen, J. Christensen, S. Lundsteen, C. Göke & M.-C. Carausu. 2016. Trends in records and contribution of nonindigenous species (NIS) to biotic communities in Danish marine waters. 44
Stranga, Y. & S. Katsanevakis. 2021. Eight years of BioInvasions Records: patterns and trends in alien and cryptogenic species records. Management of Biological Invasions 12/2: 221–239. DOI: 10.3391/mbi.2021.12.2.01, ISSN: 19898649.
Svendsen, L. M., L. van der Bijl, S. Boutrup & B. Norup. 2005a. NOVANA. Nationwide Monitoring and Assessment Programme for the Aquatic and Terrestrial Environments. Programme Description – Part 2. Denmark (DNK): 138
Svendsen, L. M., B. Norup & Lilian van der. 2005b. NOVANA. Nationwide Monitoring and Assessment Programme for the Aquatic and Terrestrial Environments. Programme Description – Part 1. Denmark (DNK): 53
The Danish Environmental Protection Agency. 2017. Action planagainst invasive species. Copenhagen: 76
Tsiamis, K., A. Palialexis, K. Stefanova, Ž. N. Gladan, S. Skejić, M. Despalatović, I. Cvitković, B. Dragičević, J. Dulčić, O. Vidjak, N. Bojanić, A. Žuljević, M. Aplikioti, M. Argyrou, M. Josephides, N. Michailidis, H. H. Jakobsen, P. A. Staehr, H. Ojaveer, M. Lehtiniemi, C. Massé, A. Zenetos, L. Castriota, S. Livi, C. Mazziotti, P. J. Schembri, J. Evans, A. G. Bartolo, S. H. Kabuta, S. Smolders, E. Knegtering, A. Gittenberger, P. Gruszka, W. Kraśniewski, C. Bartilotti, M. Tuaty-Guerra, J. Canning-Clode, A. C. Costa, M. I. Parente, A. Z. Botelho, J. Micael, J. V. Miodonski, G. P. Carreira, V. Lopes, P. Chainho, C. Barberá, R. Naddafi, A.-B. Florin, P. Barry, P. D. Stebbing & A. C. Cardoso. 2019. Non-indigenous species refined national baseline inventories: A synthesis in the context of the European Union’s Marine Strategy Framework Directive. Marine Pollution Bulletin 145: 429–435. DOI: 10.1016/j.marpolbul.2019.06.012, ISSN: 0025326X.
Tulp, I., J. Craeymeersch, M. Leopold, C. van Damme, F. Fey & H. Verdaat. 2010. The role of the invasive bivalve Ensis directus as food source for fish and birds in the Dutch coastal zone. Estuarine, Coastal and Shelf Science 90/3: 116–128.
Van der Have, T. M. & R. Lensink. 2017. Trilateral monitoring and assessment programme for alien species. Developing a proposal for the trilateral Wadden Sea area. Culemborg (NDL): 95
Waser, A. M., S. Deuzeman, A. K. wa Kangeri, E. van Winden, J. Postma, P. de Boer, J. van der Meer & B. J. Ens. 2016. Impact on bird fauna of a non-native oyster expanding into blue mussel beds in the Dutch Wadden Sea. Biological Conservation 202: 39–49. DOI: 10.1016/j.biocon.2016.08.007, ISSN: 00063207.
Busch, J. A., G. Lüerßen & F. de Jong (ed.). 2019. Trilateral Wadden Sea Management and Action Plan for Alien Species (MAPAS). (Busch, J. A., G. Lüerßen & F. de Jong). Wilhelmshaven (DEU)
Wolff, W. J. 2000. Causes of Extirpations in the Wadden Sea, an Estuarine Area in The Netherlands. Conservation Biology 14/3: 876–885. DOI: 10.1046/j.1523-1739.2000.98203.x, ISSN: 08888892.
Leppäkoski, E., S. Gollasch & S. Olenin. 2002. Oyster imports as a vector for the introduction of alien species into Northern and Western European coastal waters. in: Invasive Aquatic Species of Europe. Distribution, Impacts and Management. Springer Netherlands/Dordrecht (NDL), 193–205.
Zieritz, A., B. Gallardo, S. J. Baker, J. R. Britton, J. L. C. H. van Valkenburg, H. Verreycken & D. C. Aldridge. 2017. Changes in pathways and vectors of biological invasions in Northwest Europe. Biological Invasions 19/1: 269–282. DOI: 10.1007/s10530-016-1278-z, ISSN: 1387-3547, 1573-1464.
This report should be cited as: Büttger, H., Christoph, S., Buschbaum, C., Gittenberger, A., Jensen, K., Kabuta, S., & Lackschewitz, D. (2022). Wadden Sea Quality Status Report: Alien species (1.01). Common Wadden Sea Secretariat. https://doi.org/10.5281/zenodo.15195864
All 2022 reports may be cited collectively as: Kloepper, S., Bostelmann, A., Bregnballe, T., Busch, J.A., Buschbaum, C., Deen, K., Domnick, A., Gutow, L., Jensen, K., Jepsen, N., Luna, S., Meise, K., Teilmann, J., & van Wezel, A. (2022). Wadden Sea Quality Status Report. Common Wadden Sea Secretariat, Wilhelmshaven, Germany. Downloaded DD.MM.YYYY. qsr.waddensea-worldheritage.org