Photo: Casper Tyberg. Harbour seals.
Marine mammals
L. F. Jensen, J. Teilmann, A. Galatius, R. Pund, R. Czeck, A. Jess, U. Siebert, P. Körber, S. Brasseur
Published 2017
(Version 1.01)
1. Introduction
Three species of marine mammals are regarded as indigenous in the Wadden Sea: the harbour seal (Phoca vitulina), grey seal (Halichoerus grypus) and the harbour porpoise (Phocoena phocoena). Occasionally, other species of marine mammals visit the Wadden Sea or strand along the coastline.
Harbour seal (Phoca vitulina)
Figure 1. Harbour seal (Photo: Casper Tybjerg).
The harbour seal (see Figure 1) is the most abundant seal species in the Wadden Sea, with an estimated population size at 38,126 animals in 2017. This is similar to the numbers estimated around 1900, before hunting drastically decimated the population (Reijnders 1992). Thus, in 1975, when hunting of seals had been banned throughout the Wadden Sea, the total population was estimated at only 5,410 animals. Initially pollution hampered recovery, and the occurrence of two Phocine Distemper Virus (PDV) epizootics in 1988 and 2002 reduced the population by 57 % and 50 % respectively (Reijnders et al. 1997, Reijnders et al. 2003). Following the epizootics, the population showed strong recovery and has kept growing, although with significantly reduced growth rates during the last years.
Harbour seals are seen around haul-out sites on undisturbed sand banks that are used for breeding, moulting and resting. Breeding takes place in June and July, while the peak of the moult is in August. Tagging studies have shown that harbour seals in the Wadden Sea travel far away from the haul-outs and into the North Sea to forage (Reijnders et al. 2010). The diet varies locally and with the seasons, with generally demersal prey, including gadoids, flatfish and sand eels (Gilles et al., 2008; de la Vega et al., 2016).
Grey seal (Halichoerus grypus)
 Figure 2. Grey seals (Photo: Casper Tybjerg).
Figure 2. Grey seals (Photo: Casper Tybjerg).
Historically, grey seals (see Figure 2) were abundant in the Wadden Sea. Grey seal remains were found in deposits in the region dated between 2000 BC and 1000 AD. However, by the 16th century, the species had completely disappeared from the area probably due to severe hunting. The species gradually returned in the mid 1900s forming a small breeding colony in the German Wadden Sea and later in the Dutch part of the Wadden Sea. Since their return, the grey seal numbers have grown. During the first trilaterally coordinated survey in 2006 a total of 2,139 grey seals were counted, while in 2017 their numbers had grown to 5,445. In addition to an increase in numbers, the distribution of grey seals in the Wadden Sea has expanded to include the Danish waters. Demographic modeling has shown that part of the increase is due to an influx of grey seals from the British Isles (Brasseur et al., 2015), where the population is estimated to comprise 111,600 animals (SCOS, 2015).
Grey seals generally use the higher sandbanks in the Wadden Sea. Breeding takes place during winter (November-December) and the pup is born with a white lanugo fur protecting it from the cold. Moult peak of the adults occurs in the Wadden Sea in March and April.
Grey seals feed mainly on demersal fish species and their diet largely overlaps with that of the harbour seals. Recent studies have reported incidents of grey seals preying on harbour seals (van Neer et al., 2015) and harbour porpoises (Bouveroux et al., 2014; Leopold et al., 2015).
Harbour Porpoise (Phocoena phocoena)
Figure 3. Harbour porpoise (Photo: ITAW).
The harbour porpoise (see Figure 3) is the most common cetacean in the North Sea and the only cetacean that occurs regularly in the Wadden Sea. The total population in the North Sea is estimated at about 345,000 animals (Hammond et al., 2017). The density of harbour porpoises within the area changes seasonally. While densities increase along the coast of the Netherlands and Lower Saxony during late winter and early spring, high summer densities are observed in the eastern part of the German Bight along the coast of Denmark and Schleswig-Holstein (Hammond et al., 2002; Thomsen et al., 2007; Scheidat et al., 2011). A recent study indicates, that the area north of the German island of Borkum has gained higher importance for harbour porpoises since 2008 (Peschko et al., 2016). The distribution of harbour porpoises is mostly driven by the availability of prey species, such as gobies, herring and sand eel (Gilles et al., 2009 & 2016; Leopold, 2015).
Sexual maturity is reached at the age of 3-4 years and calves are born in June and July. Harbour porpoises feed on a variety of fish including sand eels, gadoids, clupeids and gobids and squids (Lockyer & Kinze 2003).
2. Status and trends
Overall trends
Overall trends in numbers for each species are given separately. The graphs show population development (see Figures 4 and 5).
Harbour seal (Phoca vitulina)
Harbour seals were hunted for centuries in the Wadden Sea (Jensen, 1976; Hart, 2007), and the more intensive hunt in the early 1900s critically decimated the population. Gradually, hunting was banned between 1962 in the Netherlands and 1977 in Denmark. In 1974 the population counted only 3,551 animals. From then on, the population recovered, experiencing an average growth rate of 7 % per year from 1974-1987 (Reijnders et al., 2010).
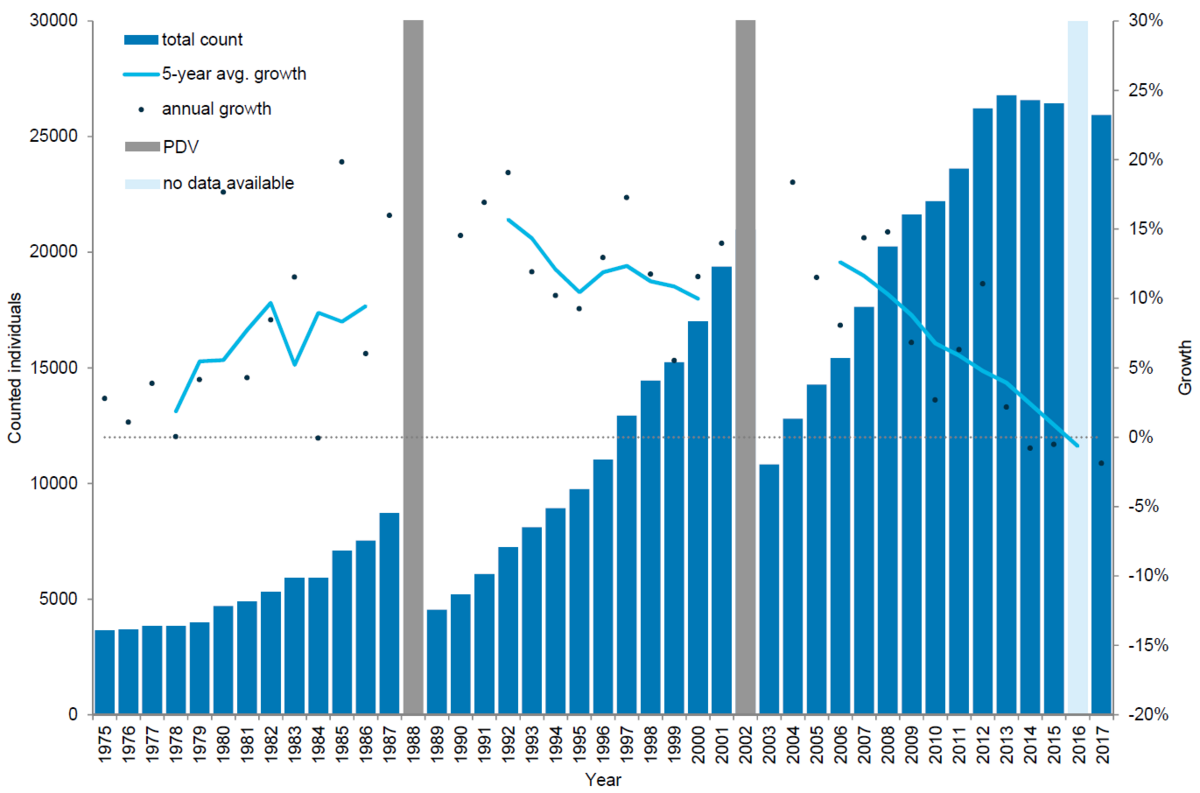 Figure 4. Harbour seal count in the Wadden Sea 1975-2017. In 2016 a trilaterally coordinated aerial survey was not possible in Lower Saxony due to weather. Thus, no total number is available for 2016.
Figure 4. Harbour seal count in the Wadden Sea 1975-2017. In 2016 a trilaterally coordinated aerial survey was not possible in Lower Saxony due to weather. Thus, no total number is available for 2016.
Two epizootics of Phocine Distemper Virus (PDV), in 1988 and 2002 respectively, had marked effects on the population of harbour seals in the Wadden Sea, reducing the population by 57 % and 50 % respectively (Reijnders et al., 1997; Reijnders et al., 2003). After the PDV epizootic in 1988 (Kennedy, 1990), the harbour seal population recovered, reaching pre-epizootic levels by 1995, and more than doubling this level by 2001. The population growth averaged 12.9 % per year in the period 1989-2002 (Abt, 2002; Reijnders et al., 2003). Clearly just after the epizootic, annual growth rates were very high, exceeding the maximum intrinsic growth rate for harbour seal populations (~12 %), possibly as a result of a changed age structure in the population (Härkönen et al., 2002). These gradually leveled off matching the intrinsic growth rate for harbour seal populations indicating that the population was growing optimally in this period.
In the period after the epizootic the distribution of the seals throughout the Wadden Sea changed. Thus, the relative importance of the Netherlands grew while that of Denmark and Schleswig-Holstein diminished. However, holding on average 39 % of the total seal population, Schleswig-Holstein remained the most important area.
In 2002, a second PDV epidemic again decimated the population (Jensen et al., 2002; Müller et al., 2004). Thus, in 2003 the population had dropped to a level similar to that of 1996. Again the recovery to pre-epizootic levels was swift: by 2007 the numbers counted surpassed the numbers in 2001. In the period 2003-2015 the population grew by 7.9 % on average with high growth rates following the epizootic, however, dropping off during the last years. In the autumn and winter of 2014 an influenza virus (Krog et al., 2015) killed a significant number of harbour seals especially in Denmark and Schleswig-Holstein. Inevitably, this affected the counts in 2015. However, an earlier decreasing trend in growth rate was evident before this epizootic, demonstrated by annual growth rates as well as 5-year average growth rates, reducing the possible variations due to e.g. weather conditions and disturbance (Teilmann et al., 2010). By 2008, population growth had dropped below the intrinsic rate and after 2013, hardly any growth was measured.
In the period after the 2002 epizootic, the distribution of the seals throughout the Wadden Sea changed again. Hence, the number of seals in the Netherlands continues to grow in importance, leading to lower numbers in German waters, especially in Schleswig-Holstein.
Since the beginning of the surveys the number of pups counted relative to the counts during the moult has grown from about 20 % to almost 30 %. This could indicate a higher pup production or a change in behaviour or age and sex composition of the animals during the moult. Clearly more pups are born in Schleswig-Holstein, where almost 50 % of all pups are observed, while less than 10 % of the births occur in Denmark, and approximately 20 % in Lower Saxony and Hamburg combined and the Netherlands.
The synchronized counts throughout the Wadden Sea have enabled the thorough analysis of the recovery of the population, including the development of their breeding success. By 2017, the total population of harbour seals in the Wadden Sea numbered about 38,126 animals. The decreasing growth rate could indicate that the harbour seal population is approaching carrying capacity. The results could also be affected by a gradual shift in behaviour, affecting the maximum numbers counted during the moult. This could for example be a result of animals undertaking longer feeding trips, thus hauling out less than in earlier years. This would match the results found with respect to the growing importance of the pups compared to the moult counts. Future studies are needed to determine the factors driving these changes.
Grey seal (Halichoerus grypus)
Historically, the grey seal occurred throughout most of Europe and the species was possibly the most abundant seal species in the Wadden Sea until around 1000 AD (Reijnders et al., 1995). With the expanding human settlement in the area, the hunting of seals increased significantly and is assumed to have caused the grey seal population to eventually disappear from the Wadden Sea in the 16th century (Reijnders et al., 1995; Härkönen et al., 2007). In the 1950s, single individuals, most likely originating from the British Isles, started appearing in the German and Dutch part of the Wadden Sea and by the 1980s a few breeding colonies were established (Scheibel & Weidel, 1988; Reijnders et al., 1995).
Since 1980 regular grey seal surveys from boats were carried out in the Netherlands and from 1988 onwards off Amrum. The first trilaterally coordinated surveys on grey seals in the Wadden Sea were carried out in the winter of 2006, during the breeding season. Before this, the population had grown exponentially by 20 % annually on average (Reijnders et al. 2006). From a total of 2,139 counted grey seals in 2006, the population doubled to 5,445 counted animals in 2017. Similarly, while 200 pups were counted in 2006, the number grew to 1,279 pups counted in 2016.
The high growth rate of the grey seal numbers in the Wadden Sea (around 15 % annually) are clearly above the theoretical maximum of about 10-11 % (Härkönen et al., 2002; Harding et al., 2007). A recent study, however, demonstrated that growth of the population is due to a large extent to annual immigration of grey seals from the UK (Brasseur et al., 2015), suggesting a high degree of connectivity among the grey seal populations in the North Sea.
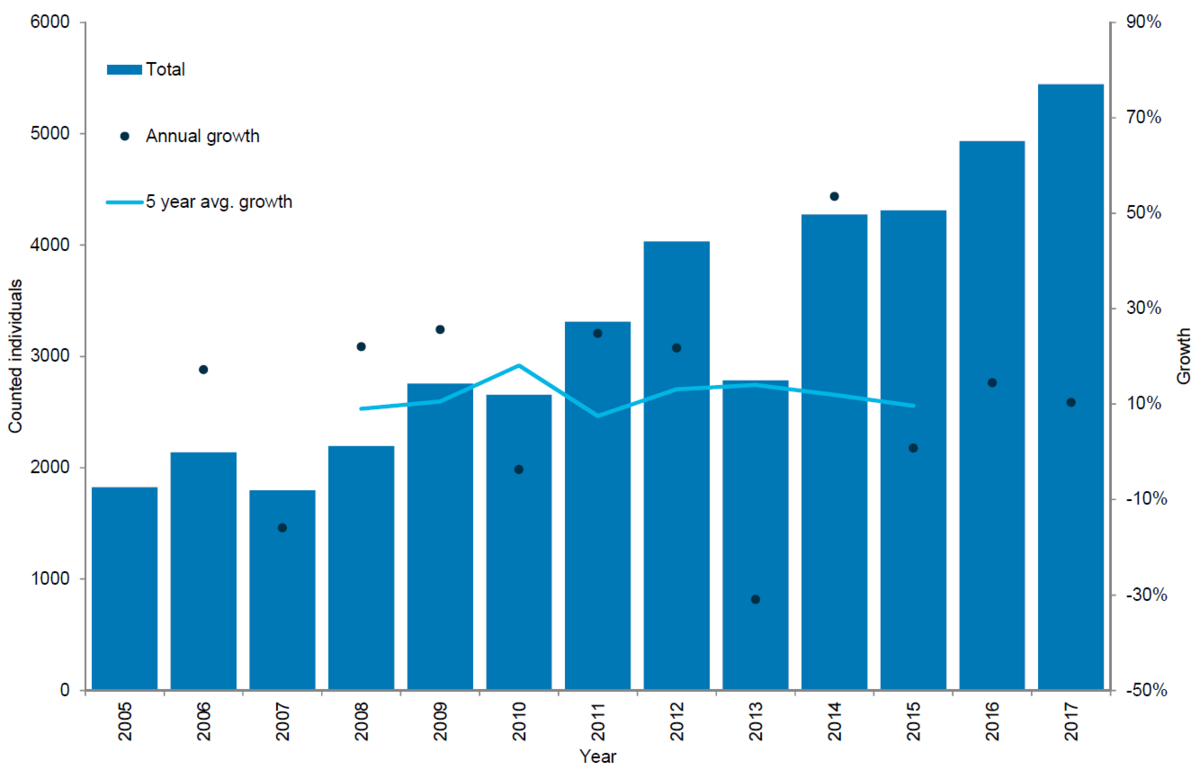 Figure 5. Grey seal count in the Wadden Sea 2005-2017.
Figure 5. Grey seal count in the Wadden Sea 2005-2017.
In recent years, the number of breeding colonies in the Dutch and German Wadden Sea has been growing (Czeck & Paul, 2008; Abt & Engler, 2009). Additionally, grey seals seem to have become increasingly abundant in the Danish Wadden Sea. Hence, dedicated surveys for grey seals in this area were initiated in 2014. In 2015 the first newborn grey seal pup since the 16th century was recorded at a sand bank in the Danish part of the Wadden Sea during a survey in December, showing that the species is now breeding throughout the entire Wadden Sea (Jensen et al., 2015).
Harbour porpoise (Phocoena phocoena)
Harbour porpoises in the Wadden Sea are believed to be part of a large North Sea population. Therefore, it is not biologically meaningful to estimate abundance in the Wadden Sea alone. To date there have been three major abundance surveys conducted in the North Sea, i.e. SCANS in 1994 and SCANS-II in 2005, and SCANS III in 2017. So far the two North Sea-wide abundance surveys revealed that there was no significant trend in the total (North Sea) abundance of porpoises. However, the average density during 2005 in survey blocks north of 56°N was about half of its density in 1994, whereas for the survey blocks south of 56°N the density was about twice the one estimated in 1994 (Hammond et al., 2015). It is likely that the majority of the changes from north to south in the North Sea are due to a displacement of animals, but a decrease in the north due to other causes such as bycatch and a true population increase in the south could also play a role.
There seems to be a seasonal pattern in the numbers observed. Data from Scheidat et al. (2006) and Gilles et al. (2008, 2009) reveal that the highest densities in the German North Sea EEZ were obtained in summer. Thus, in May 2005 harbour porpoise abundance was estimated at 64,506 animals (95 % C.I. = 36,776-127,036), while in summer 2006, an estimate of 51,551 animals (95 % CI = 27,879-98,910) was obtained. Lowest estimates were obtained in autumn (e.g., 11,573 animals in October/ November 2005).
The Gilles et al. (2008) data further showed that the spatial distribution is not homogeneous, animals have clear preferences for discrete areas. Identified hotspots were detected at Borkum Reef Ground and Sylt Outer Reef.
Similarly, the Danish monitoring program, which started in 2011, showed consistently high densities in the southern part of the Danish North Sea along the German border. Moreover, the density in the Danish Wadden Sea during August was much lower than in the offshore part of the southern Danish North Sea (Sveegaard et al., 2015).
Regional trends
Regional trends (Denmark, Germany and the Netherlands) in numbers for each species are given separately. The graphs show regional population development (Figures 6-12).
Harbour seals in the Danish Wadden Sea
In the Danish Wadden Sea a total of 2,971 harbour seals were counted during the moult count in August 2017. Corrected to take account of the number of seals in the water during the survey (Ries et al., 1998), this leads to an estimate of about 4,367 animals. Since the 2002 PDV epizootic, the number of harbour seals in the Danish Wadden Sea has increased by 9 % annually on average, though in this area growth seems to have slowed down in recent years. Thus, in the period 2011-2015 the numbers slowed down to 3 %, while in the periods 2012-2016 and 2013-2017 the population decreased slightly by 4 % and 2 % respectively.
In relation to the total population, the Danish area was home to about 11 % of all seals during the moult. Though just after the PDV epizootic in 1988 the area seemed to be of greater importance, holding 20 %, the importance of this relatively small area has remained similar throughout the study period averaging ~14 %.
Numbers of pups in the Danish part of the Wadden Sea mirror the pattern for moulting adults, showing decreasing growth rates in recent years.
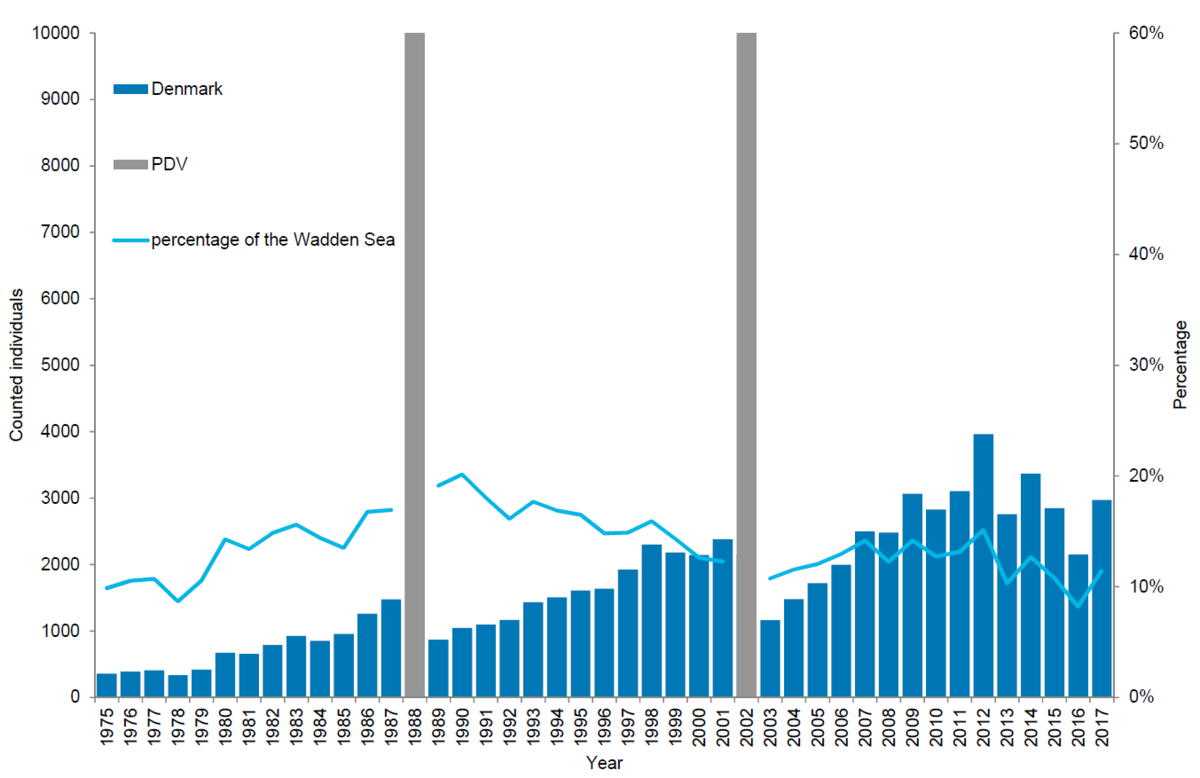 Figure 6. Harbour seal count in the Danish Wadden Sea 1975-2017.
Figure 6. Harbour seal count in the Danish Wadden Sea 1975-2017.
Harbour seals in Schleswig-Holstein
In Schleswig-Holstein, 8,834 harbour seals were counted in 2017, corresponding to an estimate of 12,986 animals after correcting to take account of the number of seals in the water during the survey. Since the 2002 PDV epizootic, the number of harbour seals in Schleswig-Holstein has increased by 6 % annually on average. Applying a five-year sliding average, reducing the effects of weather and disturbance on count numbers, this suggests that population growth in Schleswig-Holstein has remained relatively constant from 2004 to 2014 with a slight tendency towards slowing down over the period (2004-2008: 10 %, 2005-2009: 6.6 %, 2006-2010: 8.9 %, 2007-2011: 9.2 %, 2008-2012: 8.0 %, 2009-2013: 4.4 %; 2010-2014: 6.4 %), while growth has stagnated during the periods 2011-2015 (0.3 %), 2012-2016 (-4 %) and 2013-2017 (0.8 %). Pup percentage, however, has remained very high with pup to total ratios in 2013-2017 above 30 % annually.
Schleswig-Holstein is a relatively important area as it holds about 34 % of the total Wadden Sea population. The proportion of harbour seals, however, has dropped from over 45 % in the late 1970s, throughout the study period indicating that possibly here, a larger proportion of the population than in other areas had survived hunting. After hunting stopped, the seals were redistributed throughout the Wadden Sea.
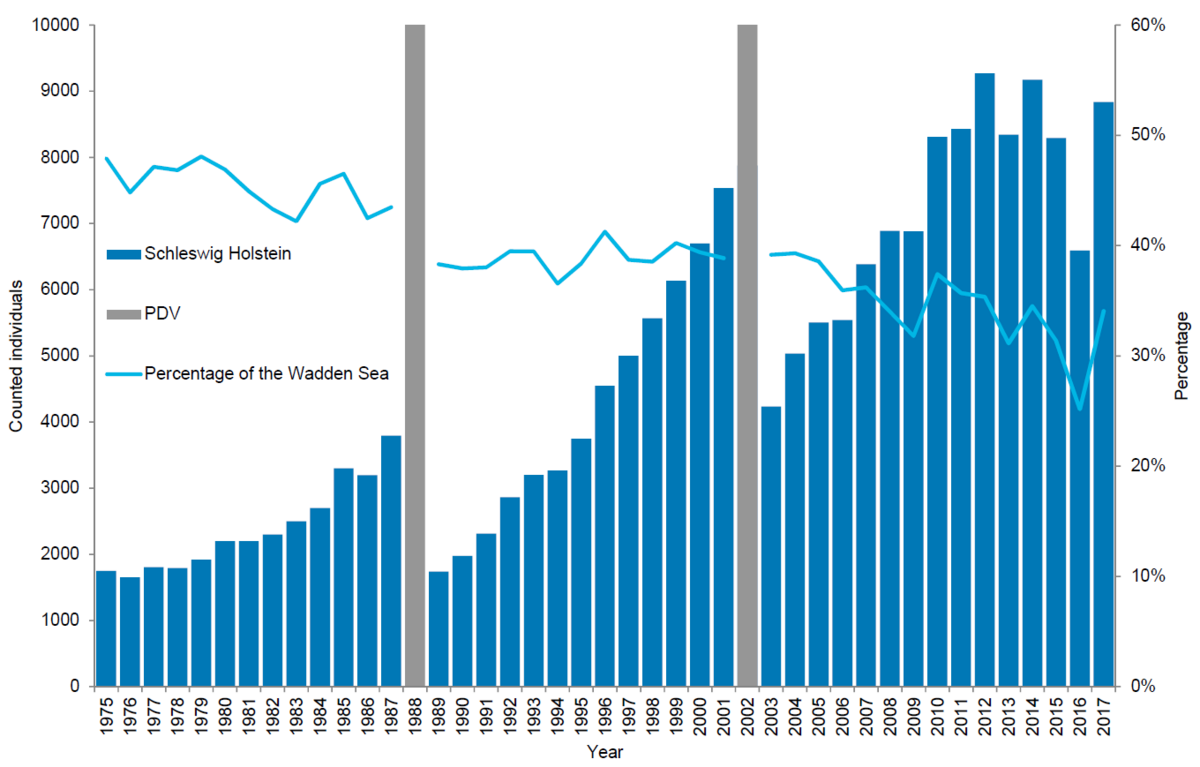 Figure 7. Harbour seal count in the Schleswig-Holstein Wadden Sea 1975-2017.
Figure 7. Harbour seal count in the Schleswig-Holstein Wadden Sea 1975-2017.
Harbour seals in Lower Saxony/ Hamburg
In Lower Saxony/ Hamburg, 7,311 harbour seals were counted in 2017 corresponding to an estimate of 10,747 animals after correcting to take account of the number of seals in the water during the survey. In Lower Saxony/ Hamburg the number of harbour seals counted during moult has increased by an average of 7.8 % annually since the last PDV epizootic in 2002. In contrast to Denmark and Schleswig-Holstein, population growth in Lower Saxony/ Hamburg does not seem to have slowed down. The relative importance of this area has remained similar throughout the study period: around 30 %, despite a drop to 20 % in 2011. Currently the numbers here represent 28 % of the population. Pup to total ratio in the 2003-2017 period average 27.5 %. Shifts in the spatial distribution of harbour seals in the different regions over time might explain the continued growth in numbers of seals in Lower Saxony/Hamburg.
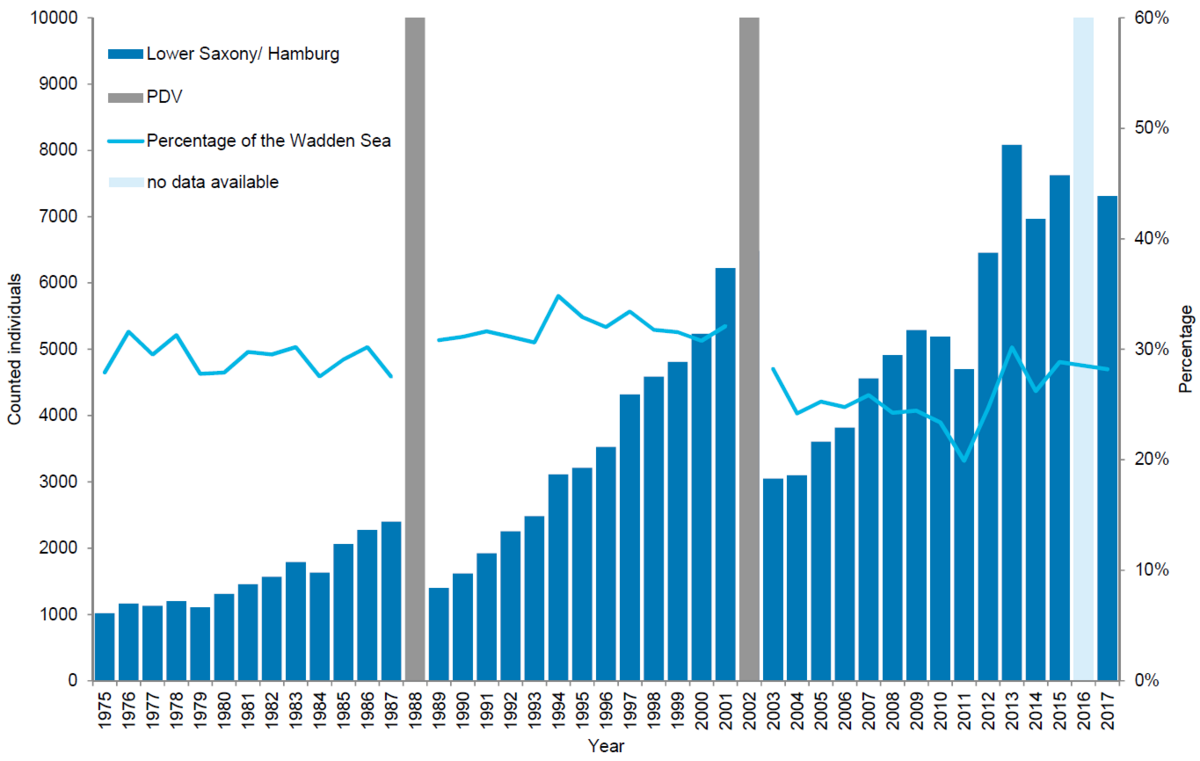 Figure 8. Harbour seal count in the Lower Saxon Wadden Sea 1975-2017. In 2016 a trilaterally coordinated aerial survey was not possible in Lower Saxony due to weather.
Figure 8. Harbour seal count in the Lower Saxon Wadden Sea 1975-2017. In 2016 a trilaterally coordinated aerial survey was not possible in Lower Saxony due to weather.
Harbour seals in the Netherlands
In the Netherlands, 6,820 harbour seals were counted in 2017 corresponding to an estimate of 10,025 animals after correcting to take account of the number of seals in the water during the survey. As in the other regions, numbers counted have shown less growth in recent years. The number of harbour seals counted during the moulting period in the Netherlands increased by an average of 9.1 % in the 2003-2017 period. This area has experienced the strongest growth in relative importance: before the epizootic of 1988 it held an average of 12 % of the population, while currently 26 % of the population is located here.
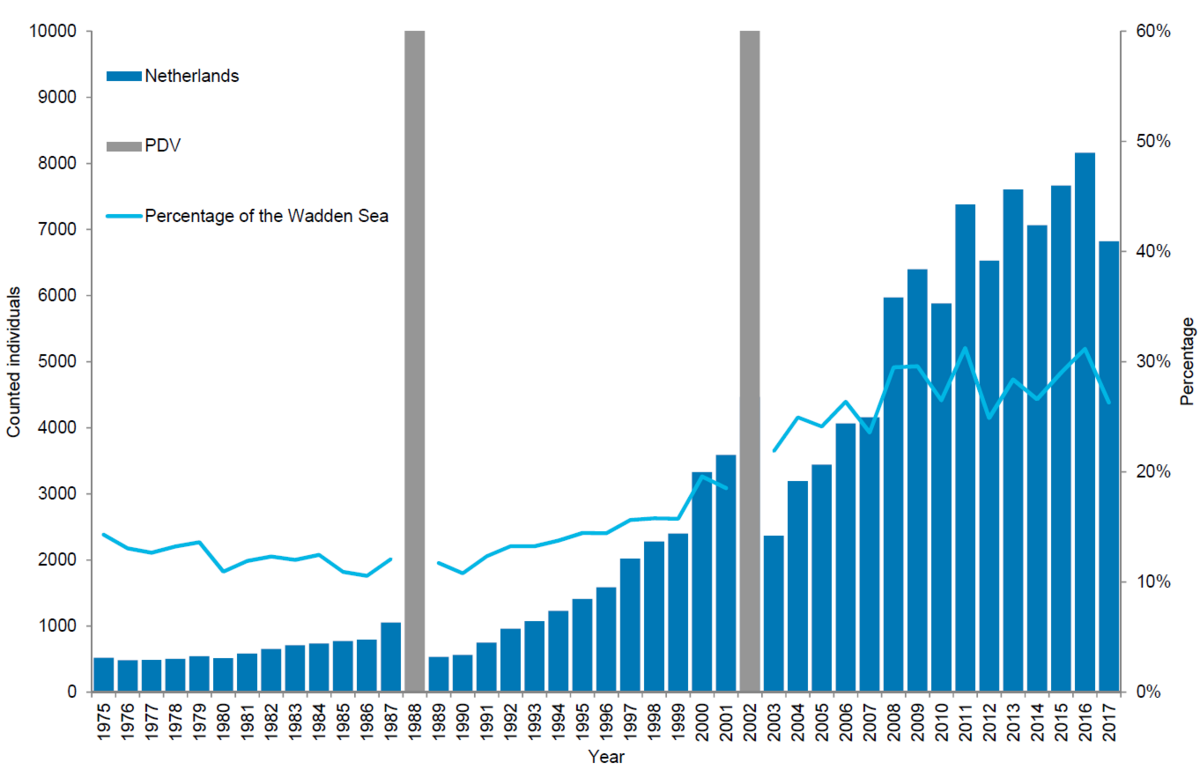 Figure 9. Harbour seal count in the Dutch Wadden Sea 1975-2017.
Figure 9. Harbour seal count in the Dutch Wadden Sea 1975-2017.
Grey seals in Denmark
Dedicated surveys for grey seals in the Danish Wadden Sea have only been carried out since 2014. Before then, grey seals were only counted during surveys for harbour seals, producing numbers not comparable to the other Wadden Sea regions. In 2015, a total of 88 greys seals were counted in the Danish Wadden Sea, while in 2017 the number had increased to 221 moulting grey seals. During a survey for grey seal pups in 2014, one newborn pup was observed, representing the first pup recorded in the region since the 16th century (Jensen et al., 2015).
Grey seals in Schleswig-Holstein and Helgoland
Most of the grey seals in the region are currently found at the island of Helgoland. Since the establishment of a breeding colony in the mid 1990s, the number of grey seals has been increasing rapidly. Thus in the 1997-2007 period the population grew by an average of 35 % annually (Abt & Engler, 2009) and in 2008, a total of 206 moulting grey seals were counted at Helgoland. In 2017 the number had increased to 616 and 287 pups were counted in the 2017 pupping season. In the Wadden Sea area of Schleswig-Holstein the number of grey seals has not increased significantly during the last eight years. Thus, in 2008 a total of 98 moulting grey seals were counted in the region, while in 2016 and 2017 the corresponding numbers were 47 and 141. The number of grey seal pups born in the Wadden Sea area of Schleswig-Holstein has seen a decline and in 2017 only one pup was observed. In comparison, the highest pup count since the onset of the trilaterally coordinated surveys totalled 40 pups (in 2010).
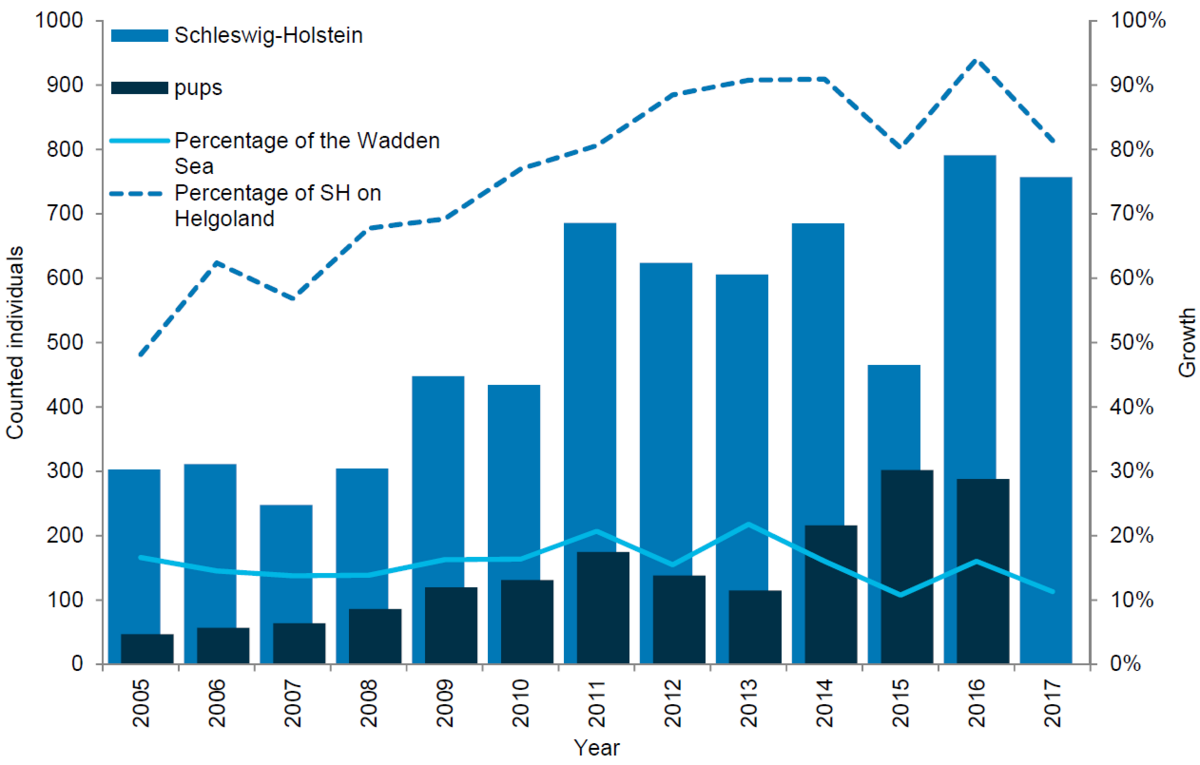 Figure 10. Grey seal count in the Schleswig-Holstein Wadden Sea 2005-2017.
Figure 10. Grey seal count in the Schleswig-Holstein Wadden Sea 2005-2017.
Grey seals in Lower Saxony/ Hamburg
In Lower Saxony/ Hamburg, the number of grey seals in terms of both moulting animals and newborn pups has generally been increasing. In 2008 a total of 174 grey seals were counted during the survey in the moulting period, while in 2017 the corresponding number had increased to 422. The increase in numbers of pups is even higher reaching 197 pups in 2017 compared to only 42 in 2006. It should be mentioned that in 2012 the counting method changed from visual to photo counts.
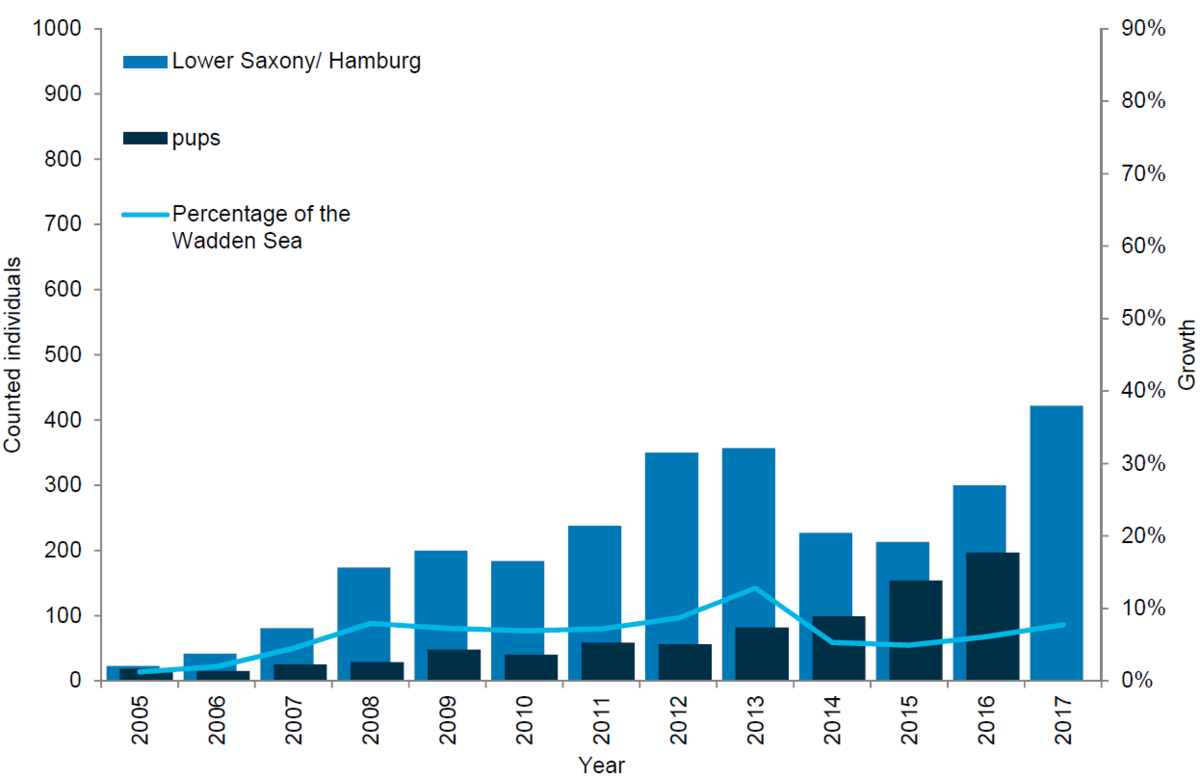 Figure 11. Grey seal count in the Lower Saxon Wadden Sea 2005-2017.
Figure 11. Grey seal count in the Lower Saxon Wadden Sea 2005-2017.
Grey seals in the Netherlands
The Netherlands represent the stronghold for the grey seals in the Wadden Sea, as the majority of the animals are found there. The number of grey seals in the Dutch Wadden Sea has been steadily increasing ever since the first breeding colony was established in the beginning of the 1980s. From 2006 to 2017 the population more than doubled, growing from 1,786 grey seals counted to 4,045 animals. In parallel to the growing numbers of moulting grey seals, the number of pups born in the region has increased. Thus in 2017 a total of 794 pups were counted in the Dutch Wadden Sea compared to just 162 pups in 2005.
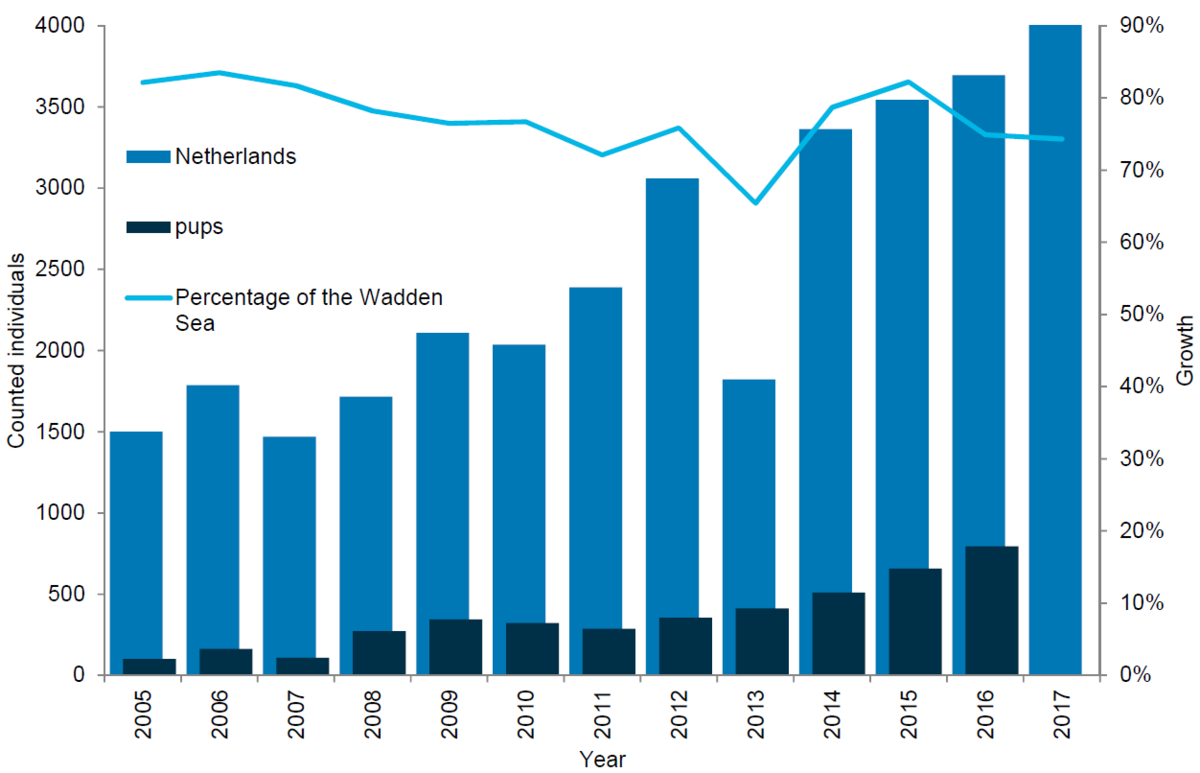 Figure 12. Grey seal count in the Dutch Wadden Sea 2005-2017.
Figure 12. Grey seal count in the Dutch Wadden Sea 2005-2017.
Harbour porpoises in the different regions
Over the past two decades an increasing number of surveys has been conducted in the southern North Sea in the context of national monitoring programs under the EU Habitats Directive and environmental impact assessments for wind farms (e.g. Geelhoed et al., 2013; Peschko et al., 2016; Sveegaard et al., 2015). All of the animals in the North Sea are considered one harbour porpoise population and the Wadden Sea is not considered a hot spot for porpoises in the southern North Sea. In the southern North Sea, a general increase in abundance has been seen for the whole region between 1994 and 2005 (Hammond et al., 2012). This trend is confirmed by surveys in German (Thomsen et al., 2006; Peschko et al, 2016) and Dutch waters (Geelhoed et al., 2013) as well as for number of calves (Peschko et al., 2016). Porpoises occur in the entire Wadden Sea area throughout the year, but significantly higher densities are seen in the eastern and northern part in summer, while the opposite picture with higher densities during winter is seen in the western part of the Wadden Sea. This suggests an unconfirmed seasonal migration of the majority of porpoises. Tracking of individuals is the only way to show seasonal movements and the first 6 porpoises have now been tagged with satellite transmitters in the Danish Wadden Sea. Preliminary results (transmitters still active) show high site fidelity with movements up to 10 km from the coastline during the fall (Teilmann et al., unpublished data).
Exchange within the Wadden Sea and between the Wadden Sea and other areas
Cetaceans are typically monitored from the air or by boats, providing for a relative index of abundance. Contrary to cetaceans, seals spend time on land, allowing annual counts providing for an estimate of the growth in numbers, pup production and distribution along the Wadden Sea coasts, at least when hauled out.
Both seals and harbour porpoise are, however, known to travel extensively between feeding areas and, for example, resting or breeding sites (Sveegaard et al., 2011; Aarts et al., 2013; Sharples et al., 2012). Therefore, distribution and densities are bound to change through time, and caution is called for defining marine mammal populations as isolated entities. There is no evidence of a separate harbour porpoise population in the southern North Sea or the Wadden Sea (e.g. Wiemann et al., 2010).
Relatively recent data collected using telemetry devices demonstrate how the animals move through their environment. Especially the more recent GPS trackers that convey their data through GSM have made it possible to collect large amounts of data on the precise movements of the seals through the area. In Dutch and Lower Saxon waters, over 200 adult and sub adult harbour seals and 65 grey seals were tracked between 2009 and 2016, providing for an overview of the distribution of the seals at sea. Typically, three types of trips can be distinguished in seal behaviour: round trips when the seal comes back to its haul out after feeding at sea; feeding trips where the seal switches haul-out, presumably to get closer to the feeding area; traveling trips where seals migrate, for example, to their breeding sites.
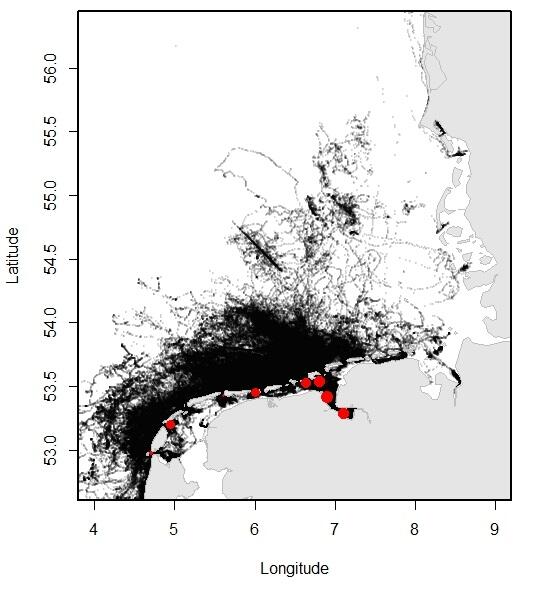
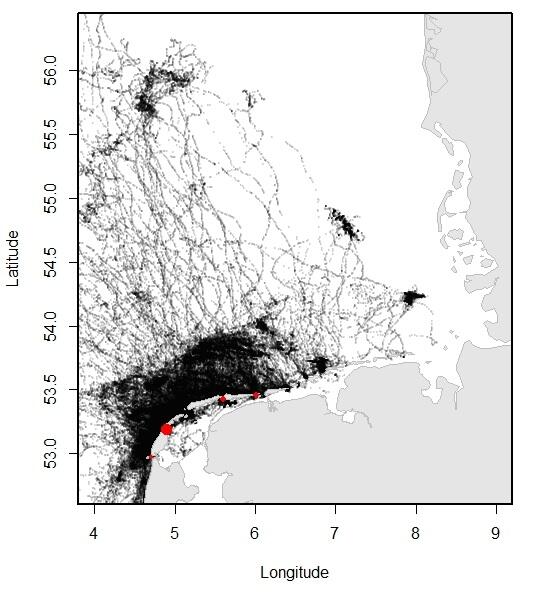 Figure 13. Left (A): Overview of the locations of harbour seals tracked from the Dutch Wadden Sea, obtained using satellite telemetry (GSM-GPS devices) between 2009 and 2016; Right (B): Overview of the locations of grey seals tracked from the Dutch Wadden Sea, obtained using satellite telemetry (GSM-GPS devices) between 2009 and 2016. Tagging locations are indicated with red dots (data published in Brasseur & Kirkwood 2016).
Figure 13. Left (A): Overview of the locations of harbour seals tracked from the Dutch Wadden Sea, obtained using satellite telemetry (GSM-GPS devices) between 2009 and 2016; Right (B): Overview of the locations of grey seals tracked from the Dutch Wadden Sea, obtained using satellite telemetry (GSM-GPS devices) between 2009 and 2016. Tagging locations are indicated with red dots (data published in Brasseur & Kirkwood 2016).
Both harbour seals and grey seals may travel far beyond the direct vicinity of the haul-out sites, which lie within the Wadden Sea (see Figure 13). Nontheless, harbour seals spend approximately 20 % of their time swimming (as opposed to hauled out) in the Wadden Sea.
Both species are known to show strong fidelity to their breeding sites (Härkönen et al., 1999; Pomeroy et al., 2000; Olsen et al., 2014). Thus, females predominantly return to the same area to breed annually and pups show natal philopatry to some extent. It is clear however, that in both species individuals may move away from these areas outside the breeding period. Especially in young individuals such movements may lead to colonisation. In case of the Wadden Sea, recolonisation probably took place by grey seals from the UK (Brasseur et al., 2015). Possibly within the Wadden Sea harbour seal population, similar processes lead to a redistribution of seals after the breeding season, with seals feeding away from their breeding sites. For example, approximately 50 % of all harbour seal pups are born in Schleswig-Holstein, while the largest relative growth is attained in the Netherlands. The few tracking results of pregnant females moving from Dutch waters to German sites to breed support this observation.
These observations provide for a first overview of the consequences of movement for the seal populations. Eventually, detailed genetic analysis and dedicated tracking experiments might shed light on how exactly the population spreads throughout its range, defining the dependency of the seals for the different areas.
Key threats to marine mammals in the Wadden Sea
Populations of harbour seals, grey seals and harbour porpoises in the Wadden Sea are regarded as viable and in no immediate risk of critical decline. However, a variety of human activities potentially influences the status of marine mammals in the Wadden Sea. These include pollution, shipping, fisheries, construction of wind farms, gravel extraction and ecotourism (Reijnders et al., 2010). Additionally, recurrent virus epizootics threaten to significantly reduce the population of harbour seals significantly. Specifically, a recent study demonstrated that only a very small proportion of the population (2 %) showed humoral immunity against Phocine Distemper Virus (PDV). Thus, upon introduction, the virus is expected to cause very high mortalities (Ludes-Wehrmeister et al., 2016).
Virus epizootics and general health development in harbour seals
Since monitoring of the harbour seal population in the Wadden Sea was introduced, recurrent virus epizootics have had a significant impact on the population in terms of both population size and distribution. In 1988 and 2002 respectively, the population was heavily decimated by two phocine distemper virus (PDV) epizootics. After both epizootics the harbour seal population recovered surprisingly quickly.
Beginning in spring 2014, a rise in harbour seal mortality was observed on Sweden's west coast, spreading to Denmark and the Wadden Sea through summer and fall. The increased mortality was caused by an epizootic of Influenza A virus subtype H10N7, not previously described in pinnipeds. Additionally, bacterial infections and pulmonary endoparasitoses played a significant role in pathogenesis. The source of the virus epidemic is currently unknown but as the virus is known to occur in water birds it is probable that it was introduced to the seal population via contact to feces from infected water birds. After the increased mortality in 2014, the harbour seal population recovered quickly.
During 20 years of systematic microbiological investigations on harbour seals, more than 11,000 bacterial isolates and fungi have been diagnosed. In total, 148 different bacteria and fungi species have been isolated, of which Escherichia coli/Escherichia coli var. haemolytica were found most frequently, followed by beta-hemolytic streptococci, Brucella pinnepidialis, Clostridium perfringens and Erysipelothrix rhusiopathiae. Bordetella bronchiseptica and Streptococcus equi subspecies zooepidemicus have almost exclusively been found during virus outbreaks and are considered secondary bacteria. As the zoonotic bacteria Brucella pinnepidialis and Erysipelothrix rhusiopathiae are frequently found, handling dead and living marine mammals should involve special care to prevent transmission to humans.
After weaning, young harbour seals are infected by a variety of parasites including, e.g., nematodes, cestodes, acanthocephalans and seal lice. Most severe lesions associated with parasites are caused by lung worms represented by Otostrongylus circumlitus and Parafilaroides gymnurus. It is assumed that, while all animals are infected when starting to feed on fish, the strongest survive the infection and attain lifelong immunity. The percentage of lung worm-infected weaned harbour seals has not increased. However, the total number of infected harbour seals has increased at a similar rate as population growth.
Compared to harbour seals, grey seals are less frequently infected by lung worms and are rarely affected by PDV. Grey seals are infected by PDV as evidenced by findings of specific antibodies in grey seals, but the infection seldom compromises the animal.
Systematic pathological investigations suggest that harbour porpoises in the Wadden Sea display a higher incidence of parasitic infections, pneumonia and chronic diseases compared to animals from regions with lower pathogenic pressure. They also reach only half their possible lifespan.
Unusual sperm whale mass stranding in 2016
In January and February 2016, a total of 30 male sperm whales (Physeter macrocephalus) stranded along the shores of the Wadden Sea and the North Sea. The stranding event began at the German Wadden Sea island of Wangerooge, when two dead sperm whales stranded on 8 January, 2016. Later, on 12 January, another dead sperm whale was found at Eversand in Germany along with an additional two carcasses drifting in the water near Helgoland. The same day five sperm whales stranded at Texel in the Netherlands. During the following days and weeks another 20 sperm whales stranded in Denmark (1), Germany (16 in total), the Netherlands (6 in total), UK (6) and France (1).
The stranding event is the largest ever reported in the North Sea. However, stranding events involving several sperm whales in the North Sea are not uncommon. In the mid-1990s three mass strandings involving sperm whales took place. On 4 December, 1997, 13 sperm whales stranded at the Danish Island of Rømø. The same year another eleven sperm whales stranded in Scotland, England, the Netherlands and Germany (Tougaard & Kinze, 1999). In the previous year (1996) a total of 25 sperm whales stranded along the North Sea coasts, with 16 animals on the Island of Rømø. Finally, in 1994/1995 a total of 15 stranded sperm whales was recorded in the area.
Strandings of sperm whales in the North Sea most often involve juvenile males, migrating in the North Atlantic between foraging areas in the Arctic and breeding grounds near the Equator. The females very seldom leave the lower latitudes. What causes the migrating sperm whales to enter the North Sea and subsequently strand is unknown, but local geological and hydrological conditions, changes in prey distribution, geomagnetism, noise pollution, seismic disturbances, climate change and diseases have all been suggested as possible causes underlying whale strandings (Hansen et al., 2014; Smeenk, 1997; Dailey et al., 2000; Scholin et al., 2000; Evans et al., 2005; Lehnert et al., 2005; Mazzariol et al., 2011; Cools et al., 2013).
3. Assessment
The harbour seal (Phoca vitulina), grey seal (Halichoerus grypus) and harbour porpoise (Phocoena phocoena) are regarded as indigenous marine mammals in the Wadden Sea. All three species are listed in Annex II of the EU Habitats Directive and special areas have been designated for their conservation. Harbour porpoises are also listed under Annex IV, which requires strict protection throughout the range of the species in European waters. Moreover, harbour seals and grey seals are listed in Annex V, which stipulates that taking in the wild and exploitation may be subject to management measures. In addition, harbour seals are protected through the Agreement on the Conservation of Seals in the Wadden Sea, or Wadden Sea Seal Agreement (WSSA), concluded under the Bonn Convention on the Conservation of Migratory Species of Wild Animals. Since the signing of the Wadden Sea Seal Agreement, grey seals have become more abundant and have been included in the Seal Management Plan elaborated under the Wadden Sea Seal Agreement (though they are not covered by the Agreement itself). The harbour porpoise is protected according to the Agreement on the Conservation of Small Cetaceans of the Baltic, North East Atlantic, Irish and North Seas (ASCOBANS).
According to the Wadden Sea Plan (Common Wadden Sea Secretariat, 2010) the targets for each marine mammal species, which are consistent with the national Conservation Objectives under the Habitats Directive as well as World Natural Heritage criterion X, are as follows:
- Viable stocks and a natural reproduction capacity of the harbour seal, including juvenile survival;
- Viable stocks and a natural reproduction capacity of the grey seal, including juvenile survival;
- Viable stocks and a natural reproduction capacity of the harbour porpoise;
- Conservation of habitat quality for the conservation of the species.
Assessment for harbour seals
The Wadden Sea population has been recovering from severe overhunting in the 20th century. The harbour seal numbers have reached the highest level since the start of the trilateral coordinated monitoring in 1975 and the population can be regarded as viable. Alternating shifts in the geographical distribution of harbour seals across the Wadden Sea caused by different proportions of seals hauling out in the different regions emphasize the importance of regarding the harbour seal population in the Wadden Sea as a whole. Pup production in 2015 was the highest ever recorded in terms of both numbers (8,439 pups) and pup percentage relative to moult counts (32 %). After high post-epizootic growth rates, population growth seems to have stagnated in recent years, indicating that the population might be approaching carrying capacity.
The most immediate threat to the population is the reoccurrence of a PDV epizootic, which killed 50 % of the population in 1988 and 2002. Pollution is presently not regarded as a major issue for the population, while bycatch has not been quantified. There is clearly a need for further assessments of the impact of these factors on population growth. The effects of underwater noise pollution due to, for instance, the construction of wind farms are unclear. Guidelines for the increasing recreation activities, including organized seal safaris, should be produced to reduce disturbance from these activities. Rehabilitation of seals in the Wadden Sea has not been reduced and is currently at a level causing concern.
Assessment for grey seals
A clear growth in the number of grey seals has been observed in the Wadden Sea area (including Helgoland), since immigrants from the UK started to recolonize the area in the mid-20th century. Grey seals have been observed in all countries though approximately 75 % of the moulting seals and 65 % of the pups are observed in the Dutch Wadden Sea. Though mechanisms of exchange have not been studied in detail, there is general consent that, rather than a separate Wadden Sea population, the grey seals might be part of a North Sea population. An important part (at least 30 %) of the growth observed is still a result of immigration from the UK.
Generally, the grey seals in the Wadden Sea breed on suboptimal sites from which the pups can be flushed off during a winter storm, causing mortality. In former years this has led to aberrant rescue efforts. In recent years, calm winters have facilitated the stabilization of these breeding sites as vegetation grew and less disruption of breeding occurred.
Though grey seals seem less susceptible to the PDV, other risks including pollution, bycatch and disturbance both at sea and on land are similar to the risks in harbour seals. Also, intense rehabilitation efforts are a concern for this species.
Assessment for harbour porpoises
The abundance of harbour porpoises in the southern North Sea has increased since the first survey in 1994. There is no specific data for the Wadden Sea and porpoises in the entire North Sea are considered to belong to one large population. However, the increasing trend in number of porpoises in all contiguous states of the southern North Sea provides the basis for a favourable conservation status of porpoises also in the Wadden Sea area.
Despite the increasing abundance, bycatch in gillnets and pollution are still considered as threats to the population, but there is clearly a need for further assessments of the impact of these factors on population growth. The effects of underwater noise pollution from the construction of wind farms, shipping and oil exploitation are under investigation and the effect on population levels is still unclear.
4. Recommendations
Recommendations for monitoring and research (based on the Seal Management Plan)
Coordinated monitoring of the harbour seal (Phoca vitulina) population in the Wadden Sea has been carried out in Denmark, Germany and the Netherlands since 1975, providing a long-term record of population trends in terms of population size and distribution of this species. By signing the Agreement on the Conservation of Seals in the Wadden Sea the three countries emphasized the importance of a coordinated monitoring program for the harbour seal population in the Wadden Sea, including annual pup and moult counts. On this basis the Seal Management Plan 2012-2016 defines a minimum effort of five aerial surveys each year – three during pupping season and 2 surveys during the moulting period.
As demonstrated by Reijnders et al. (2010) changes in breeding phenology of harbour seals in the Wadden Sea have taken place over the past years. Thus, the mean birth date of seal pups has shifted by -0.71 days per year since the early 1970s. As a result, the birth peak occurred 25 days earlier in 2009 than in the 1970s. Importantly, the shift was continual but not constant over the period studied. Similarly, investigating individual-based estimates of birth dates, Cordes & Thompson (2013) observed an advance in the timing of pupping in a population of harbour seals in Scotland over a period of 25 years. Hence, it has been demonstrated that timing of pupping in harbour seals is a variable trait under the likely influence of factors such as maternal nutritional status, population demography and density or possibly global warming (Abt, 2010). Accordingly, a monitoring program should accommodate inter-annual variation in the timing of pupping.
It is reasonable to expect that the observed changes in breeding phenology are accompanied by changes in the timing of the moult. On this basis it is recommended that three extra coordinated survey flights be conducted every fifth year: two extra flights during the pupping season in order to track changes in breeding phenology and one extra survey flight during the moulting season to accommodate temporal changes in timing of the moult.
Scientific fields where further research and monitoring are needed include:
- Feeding ecology of marine mammals at both temporal and spatial scale, including interspecific competition;
- Habitat use at sea based on telemetry of all three species of marine mammals;
- Effects from anthropogenic activities on marine mammals in the Wadden Sea;
- Effects from diseases and contaminants on the health status of marine mammals including assessments of development and effects from new pollutants with physiological impact;
- The use of marine mammals as bio-indicators of environmental conditions.
Figure 14. Aerial survey in the Wadden Sea (Photo: Casper Tybjerg).
Recommendations for management
Disturbance
It is recommended to define special protected zones within the Wadden Sea for harbour and grey seals haul-outs and regularly adapt these according to changes in natural conditions, e.g. relocation of sand banks. This would help ensure species protection for the seals to breed, moult and rest.
As seals spend the majority of their time at sea (>80 %), it is recommended to plan and adapt human activities at sea according to the needs of the seals. This requirement has become more urgent in the light of the growth of off-shore activities such as wind farming, mining for oil and gas, fisheries and aquaculture, military activities and shipping.
Rehabilitation
A complete stop of the rehabilitation of pinnipeds in the Wadden Sea, i.e. the temporary rearing of a wild animal with the intent of releasing it back into its natural habitat, is recommended.
Rehabilitation as a means of supporting the wild populations is not necessary as population numbers are high and well above the limit of 1,000 individuals that has been suggested to ensure a probability of persistence above 99 % over a 200-year period (Olsen et al., 2014).
Rehabilitation causes concern for a number of reasons. Of highest concern is the risk of introducing diseases into the wild populations. Possible routes for disease transmission to seals in rehabilitation include terrestrial mammals at the centers and the holding of other species of pinnipeds in the same facility (Hastings et al.; 1989, Stamper et al., 1998). Pathogens are also at risk of being modified at rehabilitation centers due to contact with new hosts or changes in the environment (Stoddard et al. 2009). Finally, there is the concern that rehabilitation could have genetic consequences, which might change the genetic makeup of the population by counteracting the process of natural selection and host-pathogen co-evolution (Wilkinson and Worthy, 1999; Moore et al., 2007).
Consequently, Denmark, Germany and the Netherlands have agreed to reduce the number of rehabilitated seals in the Wadden Sea to a minimum. Specifically, the intent is expressed in the Agreement on the Conservation of Seals in the Wadden Sea as well as the Ministerial Declaration of the seventh Trilateral Governmental Conference in Leeuwarden 1994. Accordingly, Denmark discontinued rehabilitation in 1995, but rehabilitation of seals is still being carried out in Germany and the Netherlands and the numbers of animals rehabilitated is still rising.
Fishery management
Potentially, fishery has both direct and indirect impacts on marine mammals through risk of entanglement and drowning in gillnets and increased risk of starvation due to depletion of stocks of important prey species targeted by commercial fisheries (Mac Leod et al., 2007).
Deterrent devices such as pingers attached to gillnets may significantly reduce bycatch of harbour porpoises but are also associated with problems. For instance, sound emission increases underwater noise and the use of pingers inside protected areas risks driving animals out of the area instead of protecting them inside.
It is recommended to avoid gillnet fishery at least inside zones protected as FFH-areas, although gillnet fishery is not considered a major problem within the Wadden Sea. Similarly, it is recommended to close specific areas with a high density of marine mammals for commercial fishery (Herr et al., 2009) to avoid direct competition between fishery and marine mammals.
Sand extraction management
It is recommended to avoid sand extraction in the Wadden Sea. Sediment removal should be regulated in terms of area, time and quantity by Habitats Directive assessments. Careful site selection and regulated timing are essential in order to protect marine mammals.
Previously, only an environmental impact assessment (EIA) had to be carried out for the approval procedure concerning sand and gravel extraction. Thus, a number of extraction plans were approved in Natura 2000 protected areas into the 2000s without having been subject to adequate assessment. Since then, the assessment standards applied in approval procedures for sediment extraction in the North Sea and Baltic Sea have been significantly improved to limit the adverse effects of sand and gravel extraction. Today, extraction of mineral resources near and within Natura 2000 sites can only be approved after submission of an additional Habitats Directive assessment. In addition, documentation on statutory habitat conservation, species conservation and impact mitigation has to be presented for extraction plans outside of protected areas. Completed and ongoing research has significantly added to our knowledge of matters such as the distribution of protected species and habitats, facilitating the identification of potential adverse effects.
It is recommended that, upon approval, a minimum separation of 500 to 750 m should be observed to ensure that protected habitats are not harmed. Where necessary, sediment removal can be regulated in terms of area, time and quantity. Filter techniques and targeted and controlled discharge of surplus material after washing or screening could help reduce turbidity plumes.
To avoid the adverse impacts of sand and gravel extraction using cutter suction dredgers, and notably the formation of craters with ‘dead’ zones, this method is now no longer used in German waters.
Human activities with impact on marine mammals and their prey
Several human activities potentially have an impact on marine mammals beside fishery and sand extraction. For instance, underwater noise pollution has increased during the last decades. Activities such as pile driving during wind farm construction produce noise levels detrimental to marine mammals. Several regulations have been directed at reducing underwater noise, including the use of two indicators for underwater noise in describing Good Environmental Status under the Marine Strategy Framework Directive.
It is recommended to reduce the level of underwater noise by developing new technologies. This applies to ship engines, sonar techniques, exploration techniques and others. This recommendation is not limited to the Wadden Sea. For example, the use of bubble curtains around a punctual noise source, e.g. pile driving and underwater explosions, help reduce noise and should be made mandatory.
Disease monitoring
Investigations of the health of marine mammals in the Wadden Sea are vital to understanding the impact of anthropogenic activities as well as general health development. The health assessment should include clinical and morpho-patholological, microbiological, virological, parasitological, serological and reproductive investigations on dead (stranded and bycaught) individuals as well as animals captured alive. The number of investigated marine mammals should allow a representative evaluation of marine mammal population health in the Wadden Sea.
5. Summary
Marine mammals in the Wadden Sea, i.e. harbour seals, grey seals and harbour porpoises, are at the top of the food chains and their population status, distribution, behaviour and health provide good indicators of ecosystem health. For this reason, harbour seal population monitoring has been carried out since 1975 and since 1991 in the framework of the Agreement on the Conservation of Seals in the Wadden Sea under the Bonn Convention. With the return of the grey seal to the Wadden Sea marine mammal fauna, the Seal Management Plan has been extended to cover this species as well. The harbour porpoises in the Wadden Sea are believed to be part of a large North Sea population and thus no monitoring of population abundance within the Wadden Sea alone is carried out.
After having experienced dramatic declines mainly due to hunting and pollution, populations of marine mammals in the Wadden Sea are now considered viable. Thus, in 2017 the harbour seal population was estimated at 38,100 animals and might be approaching carrying capacity. In 2014 the harbour seal population was struck by an Influenza A H10N7 epizootic. However, in spite of the increased mortality, the population remained stable. Temporal shifts of the spatial distribution of harbour seals throughout the Wadden Sea emphasize the need to manage the Wadden Sea harbour seals as one coherent population.
After being completely extirpated from continental Europe mainly due to hunting, the grey seal returned to the Wadden Sea in the 1960s and has experienced high population growth rates. Thus, in 2016 a total of 4,936 grey seals was counted in the Wadden Sea. The high population recovery is partly fueled by immigration of grey seals from the British Isles, yet 1,113 grey seal pups were counted in the Wadden Sea in 2015.
The total population of harbour porpoises in the North Sea is estimated at about 345,000 animals. The three North Sea-wide abundance surveys that were carried out in 1994, 2005 and 2016 respectively indicate only a slight change in abundance of porpoises over time. However, average density during 2005 in survey blocks north of 56°N was only about half of its density in 1994, whereas in survey blocks south of 56°N, the density seems to have doubled.
Although the populations have experienced steady growth in recent years, the status of marine mammals in the Wadden Sea is potentially influenced by human activities in the area. These activities include both direct impacts from activities such as wind farm construction, sand extraction and tourism and indirect impacts, influencing for instance food resources such as fisheries. Although pollution and bycatch are presently not regarded as major threats to the populations, knowledge of their impacts is scarce.
About the authorsL. F. Jensen1, J. Teilmann2, A. Galatius2, R. Pund3, R. Czeck4, A. Jess5, U. Siebert6, P. Körber7, S. Brasseur8 1 Department of Chemistry and Bioscience, Aalborg University, Fredrik Bajers Vej 7, DK-9220 Aalborg, DK 2 Aarhus University, Marine Mammal Research, Department of Bioscience, Frederiksborgvej 399, 4000 Roskilde, DK 3 LAVES, Schleusenstr. 1, 27472 Cuxhaven, DE 4 National Park Authority Wadden Sea Lower Saxony, Virchowstr. 1, 26382 Wilhelmshaven, DE 5 National Park Authority Wadden Sea Schleswig-Holstein, Schlossgarten 1, 25832 Tönning, DE 6 Institute for Terrestrial and Aquatic Wildlife Research (ITAW), Werftstr. 6, 25761 Büsum, DE 7 National Park Authority Wadden Sea Hamburg, Neuenfelder Straße 19, 21109 Hamburg, DE 8 Institute for Marine Resources & Ecosystem Studies (IMARES), Postbus 167, 1790 AD Den Burg/Texel, NL |
References
Aarts G, Brasseur S, Geelhoed S, van Bemmelen R & Leopold M (2013) Grey and harbour seal spatiotemporal distribution along the Dutch West coast. IMARES Wageningen UR.
Abt K (2010) Earlier pupping in harbour seals likely driven by climate – rather than by fisheries. Response to Reijnders P.J.H, Brasseur S.M.J.M. & Meesters E.H.W.G. (2010) Earlier pupping in harbour seals, Phoca vitulina. Biology Letters, 6, 854-857.
Abt KF (2002) Phänologie und Populationsdynamik des Seehundes Phoca vitulina im Wattenmeer: Grundlagen zur Messung von Statusparametern. Ph.D thesis, Universität Kiel. Büsum, Berichte des Forschungs- und Technologiezentrums der Universität Kiel Nr. 24, pp. 117.
Abt K & Engler J (2009) Rapid increase of the grey seal (Halichoerus grypus) breeding stock at Helgoland. Helgoland Marine Research 63, 177–180.
Bouveroux T, Kiszka JJ, Heithaus MR, Jauniaux T & Pezeril S (2014) Direct evidence for gray seal (Halichoerus grypus) predation and scavenging on harbour porpoises (Phocoena phocoena). Marine Mammal Science, 30,1542-1548.
Brasseur SMJM. & Kirkwood RJ (2016) Seal monitoring and evaluation for the Gemini offshore windpark: T-construction - 2015 report. C043/16 pp. 52. IMARES, Den Burg.
Brasseur SMJM, van Polanen Petel TD, Gerrodette T, Meesters EHWG, Reijnders PJH & Aarts G (2015) Rapid recovery of Dutch gray seal colonies fueled by immigration. Marine Mammal Science, 31, 405-426.
Common Wadden Sea Secretariat (2010) Wadden Sea Plan 2010. Eleventh Trilateral Governmental Conference on the Protection of the Wadden Sea. Common Wadden Sea Secretariat, Wilhelmshaven, Germany.
Cools P, Haelters J, Lopes dos Santos Santiago G, Claeys G, Boelens J, Leroux-Roels I, Vaneechoutte M & Deschaght P (2013). Edwardsiella tarda sepsis in a live-stranded sperm whale (Physeter macrocephalus). Veterinary Microbiology, 166, 311-315.
Cordes LS & Thompson PM (2013) Variation in breeding phenology provides insights into drivers of long-term population change in harbour seals. Proceedings of the Royal Society B, 280: 20130847.
Czeck R & Paul M (2008) Grey seals – a homecoming species in the Wadden Sea. Senckenbergiana Maritima, 38, 143–146.
Dailey MD, Gulland FMD, Lowenstine LJ, Silvagni P & Howard D (2000). Prey, parasites and pathology associated with the mortality of a juvenile gray whale (Eschrichtius robustus) stranded along the northern California coast. Diseases of Aquatic Organisms, 31, 111-117.
de la Vega C, Lebreton B, Siebert U, Guillou G, Das K, Asmus R & Asmus H (2016) Seasonal Variation of Harbour Seal's Diet from the Wadden Sea in Relation to Prey Availability. PLoS ONE 11(5): e0155727.
Evans K, Thresher R, Warneke RM, Bradshaw CJ, Pook M, Thiele D & Hindell MA (2005). Periodic variability in cetacean strandings: Links to large-scale climate events. Biology Letters, 1, 147-150.
Geelhoed SCV, Scheidat M, van Bemmelen RSA & Aarts G (2013) Abundance of harbour porpoises (Phocoena phocoena) on the Dutch Continental Shelf, aerial surveys in July 2010–March 2011. Lutra, 56 (1), 45–57.
Gilles A, Andreasen H, Müller S & Siebert U (2008) Nahrungsökologie von marinen Säugetieren und Seevögeln für das Management von NATURA 2000 Gebieten. Endbericht für das Bundesamt für Naturschutz.
Gilles A, Scheidat M & Siebert U (2009) Seasonal distribution of harbour porpoises and possible interference of offshore wind farms in the German North Sea. Marine Ecology Progress Series, 383, 295-307.
Gilles A, Viquerat S, Becker EA, Forney KA, Geelhoed SCV, Haelters J, Nabe-Nielsen J, Scheidat M, Siebert U, Sveegaard S, van Beest FM, van Bemmelen R & Aarts G (2016). Seasonal habitat-based density models for a marine top predator, the harbour porpoise, in a dynamic environment. Ecosphere, 7(6): e01367.
Hammond PS, Berggren P, Benke H, Borchers DL, Collet A, Heide-Jørgensen MP, Heimlich S, Hiby AR, Leopold MF & Øien N (2002) Abundance of the harbour porpoise and other cetaceans in the North Sea and adjacent waters. Journal of Applied Ecology, 39, 361-376.
Hammond PS, Lacey C, Gilles A, Viquerat S, Börjesson P, Herr H, Macleod K, Ridoux V, Santos MB, Scheidat M, Teilmann J, Vingada J, Øien N (2017) Estimates of cetacean abundance in European Atlantic waters in summer 2016 from the SCANS-III aerial and shipboard surveys; https://synergy.st-andrews.ac.uk/scans3/files/2017/05/SCANS-III-design-based-estimates-2017-05-12-final-revised.pdf
Hammond PS, Macleod K, Berggren P, Borchers DL, Burt ML, Cañadas A, Desportes G, Donovan GP, Gilles A, Gillespie D, Gordon J, Hedley S, Hiby L, Kuklik I, Leaper R, Lehnert K, Leopold M, Lovell P, Øien N, Paxton C, Ridoux V, Rogan E, Samarra F, Scheidat M, Sequeira M, Siebert U, Skov H, Swift R, Tasker ML, Teilmann J, Van Canneyt O & Vázquez JA. (2013). Cetacean abundance and distribution in European Atlantic shelf waters to inform conservation and management. Biological Conservation, 164, 107-122.
Hansen MS, Alstrup AKO, Hansen JH, Al-Sabi MNS, Nonnemann B, Jensen LF, Hedayat A & Jensen TH (2016) Stranding of two sperm whales (Physeter macrocephalus) in the “North Sea Trap” at Henne Strand, Denmark. Aquatic Mammals, 42(1), 35-41.
Harding KC, Härkönen T, Helander B & Karlsson O (2007) Status of Baltic grey seals: Population assessment and extinction risk. NAMMCO Scientific Publications, 6, 33-56.
Härkönen T, Harding KC & Heide-Jørgensen MP (2002) Rates of increase in age-structured populations: a lesson from the European harbour seals. Canadian Journal of Zoology 80, 1498–1510.
Härkönen T, Harding KC & Lunneryd SG (1999) Age- and sex-specific behaviour in harbour seals Phoca vitulina leads to biased estimates of vital population parameters. Journal of Applied Ecology, 36, 825-841.
Härkönen T, Brasseur S, Teilmann J, Vincent C, Dietz R, Abt K & Reijnders P (2007) Status of grey seals along mainland Europe from the Southwestern Baltic to France. NAMMCO Scientific Publications, 6, 57-68.
Hart, t.P. (2007) Zeehondenjacht in Nederland 1591-1962 (Seal hunting in the Netherlands, 1591-1962). PhD thesis, Free University Amsterdam.
Hastings BE, Lowenstine LJ, Gage LJ & Munn RJ (1989) An epizootic of seal pox in pinnipeds at a rehabilitation center. Journal of Zoo and Wildlife Medicine, 20, 282-290.
Jensen, A., Søndergaard N-O, Hansen EB (1976) Occurrence of seals and seal hunting in Denmark. Danish Review of Game Biology, 10, 1-20.
Jensen LF, Galatius A & Teilmann J (2015) First report on a newborn grey seal pup (Halichoerus grypus) in the Danish Wadden Sea since the 16th Century. Marine Biodiversity Records, 8, e131.
Jensen T, van de Bildt M, Dietz HH, Andersen TH, Hammer AS, Kuiken T & Osterhaus A (2002) Another phocine distemper outbreak in Europe. Science, 297, 209.
Kennedy S (1990) A review of the 1988 European seal epizootic. Veterinary Record, 127, 563-567.
Krog JS, Hansen MS, Holm E, Hjulsager CK, Chriél M, Pedersen K, Andresen LO, Abildstrøm M, Jensen TH & Larsen LE (2015) Influenza A(H10N7) Virus in Dead Harbour Seals, Denmark. Emerging Infectious Diseases, 21, 684-687.
Lehnert K, Raga J A & Siebert U (2005). Macroparasites in stranded and bycaught harbour porpoises from German and Norwegian waters. Diseases of Aquatic Organisms, 64, 265-269.
Leopold MF, Begeman L, van Bleijswijk JDL, IJsseldijk LL, Witte HJ & Gröne A (2015) Exposing the grey seal as a major predator of habour porpoises. Proceedings of the Royal Society B, Biological Sciences, 282: 20142429.
Leopold, Maarten Frederik. Eat and be eaten: porpoise diet studies. Diss. Wageningen University, 2015.
Lockyer C & Kinze C (2003) Status, ecology and life history of harbour porpoise (Phocoena phocoena), in Danish waters. NAMMCO Scientific Publications, 5, 143-176.
Ludes-Wehrmeister E, Dupkea C, Harder TC, Baumgärtner W, Haas L, Teilmann J, Dietz R, Jensen LF & Siebert U (2016) Phocine distemper virus (PDV) seroprevalence as predictor for future outbreaks in harbour seals. Veterinary Microbiology, 183, 43–49.
Mazzariol S, Di Guardo G, Petrella A, Marsili L, Fossi CM, Leonzio C, Zizzo N, Vizzini S, Gaspari S, Pavan G, Podestà M, Garibaldi F, Ferrante M, Copat C, Traversa D, Marcer F, Airoldi S, Frantzis A, De Bernaldo Quirós Y, Cozzi B & Fernández A (2011) Sometimes sperm whales (Physeter macrocephalus) cannot find their way back to the high seas: A multidisciplinary study on a mass stranding. PLOS ONE, 6(5), e19417.
Meesters E, Reijnders P, Brasseur S, Siebert U, Stede M, Tougaard S & Härkönen T (2007) An effective survey design for harbour seals in the Wadden Sea: tuning trilateral Seal Agreement and EU-habitat Directive requirement. In: The meeting of the Trilateral Working Group TWG 07/1, Delfzijl, The Netherlands, 18-19 April, The Netherlands. Wilhelmshaven: Trilateral Working Group 2007.
Moore M, Early G, Touhey K, Barco S, Gulland F & Wells R (2007) Rehabilitation and release of marine mammals in the United States: Risks and benefits. Marine Mammal Science, 23, 731-750.
Müller G, Wohlsein P, Beineke A, Haas L, Greiser-Wilke I, Siebert U, Fonfara S, Harder T, Stede M, Gruber AD & Baumgärtner (2004) Phocine Distemper in German Seals. Emerging Infectious Diseases, 10, 723-725.
Olsen MT, Andersen LW, Dietz R, Teilmann J, Härkönen T & Siegismund HR (2014) Integrating genetic data and population viability analyses for the identification of harbour seal (Phoca vitulina) populations and management units. Molecular Ecology, 23, 815-831.
Peschko V, Ronnenberg K, Siebert U & Gilles A (2016). Trends of Harbour Porpoise (Phocoena phocoena) Density in the Southern North Sea. Ecological Indicators, 60, 174-183.
Pomeroy PP, Twiss SD & Redman P (2000) Philopatry, site fidelity and local kin associations within grey seal breeding colonies. Ethology, 106, 899-919.
Reijnders PJH (1992) Retrospective population analysis and related future management perspectives for the harbour seal Phoca vitulina in the Wadden Sea. In Dankers N, Smit CJ & Scholl M (eds.) Proceedings of the 7th International Wadden Sea Symposium, Ameland, 22-26 October. October 1990.
Reijnders PJH, Abt KF, Brasseur SMJM, Tougaard S, Siebert U & Vareschi E (2003) Sense and sensibility in evaluating aerial counts of harbour seals in the Wadden Sea. Wadden Sea Newsletter, 28, 9-12.
Reijnders PJH, Brasseur SMJM, Abt K, Siebert U, Stede M & Tougaard S (2006) Aerial surveys of harbour seals and grey seals in the Wadden Sea in 2006. Wadden Sea Newsletter, 1, 9-11.
Reijnders PJH, Brasseur SMJM & Brinkman AG (2003) The phocine distemper virus outbreak of 2002 amongst harbour seals in the North Sea and Baltic Sea: spatial and temporal development, and predicted population consequences. In: Management of North Sea harbour and grey seal populations. Proceedings of the international Symposium at EcoMare, Texel, The Netherlands, November 29-30, 2002. Wadden Sea Ecosystem No. 17. Wadden Sea Secretariat, Wilhelmshaven, Germany, 19-25.
Reijnders PJH, Brasseur SMJM. & Meesters EHWG. (2010) Earlier pupping in harbour seals, Phoca vitulina. Biology Letters, 6, 854-857.
Reijnders PJH, Brasseur SMJM, Tougaard S, Siebert U, Borchardt T & Stede M (2010) Population development and status of harbour seals (Phoca vitulina) in the Wadden Sea. NAMMCO Scientific Publications, 8, 95-106.
Reijnders PJH, Ries EH, Tougaard S, Nørgaard N, Heidemann G, Schwartz J, Vareschi E & Traut IM (1997) Population development of harbour seals Phoca vitulina in the Wadden Sea after the 1988 virusepizootic. Journal of Sea Research, 38, 161-168.
Reijnders PJH, van Dijk J & Kuiper D (1995) Recolonization of the Dutch Wadden Sea by the grey seal Halichoerus grypus. Biological Conservation 71, 231–235.
Ries EH, Hiby LR & Reijnders PJH (1998) Maximum likelihood population size estimation of harbour seals in the Dutch Wadden Sea based on a mark-recapture experiment. Journal of Applied Ecology, 35, 332-339.
Sharples RJ, Moss SE, Patterson TA & Hammond PS (2012) Spatial variation in foraging behaviour of a marine top predator (Phoca vitulina) Determined by a Large-Scale Satellite Tagging Program. PLoS ONE, 7, e37216.
Scheibel W & Weidel H (1988) Vorkommen von Kegelrobben (Halichoerus grypus Fabricius, 1791, Phocidae, Pinnipedia) in Schleswig-Holstein. Zoologischer Anzeiger 220, 65–70.
Scheidat M, Tougaard J, Brasseur S, Carstensen J, Petel TVP, Teilmann J & Reijnders P (2011) Harbour porpoises (Phocoena phocoena) and wind farms: a case study in the Dutch North Sea. Environmental Research Letters, 6: 025102.
SCOS, Main Advice (2015) Scientific Advice on Matters Related to the Management of Seal Populations: 2015 Reports of the UK Special Committee on Seals, http://smub.st-andrews.ac.uk.
Smeenk C (1997). Strandings of sperm whales Physeter macrocephalus in the North Sea: History and patterns. Bulletin de L’Institut Royal des Sciences Naturelles de Belgique, Biologie 67-Supplement, 15-28.
Sonntag RP, Benke H, Hiby AR, Lick R & Adelung D (1999) Identification of the first harbour porpoise (Phocoena phocoena) calving ground in the North Sea. Journal of Sea Research, 41, 225–232.
Stamper MA, Gulland FMD & Spraker T (1998) Leptospirosis in rehabilitated pacific harbour seals from California. Journal of Wildlife Diseases, 34, 407-410.
Stoddard RA, Atwill ER, Conrad PA, Byrne BA, Jang S, Lawrence J, McCowan B & Gulland FMD (2009) The effect of rehabilitation of northern elephant seals (Mirounga angustirostris) on antimicrobial resistance of commensal Escherichia coli. Veterinary Microbiology, 133, 264-271.
Sveegaard S, Galatius A & Teilmann J (2015). Havpattedyr – sæler og marsvin. In Hansen JW (ed.) Marine områder 2014: NOVANA. Aarhus Universitet, DCE – Danish Centre for Environment and Energy, p. 86-97. Scientific report, no. 167.
Sveegaard S, Teilmann J, Tougaard J, Dietz R, Mouritsen KN, Desportes G & Siebert U (2011) High-density areas for harbour porpoises (Phocoena phocoena) identified by satellite tracking. Marine Mammal Science, 27, 230-246.
Teilmann J, Riget F & Härkönen T (2010) Optimising survey design for Scandinavian harbour seals: Population trend as an ecological quality element. ICES Journal of Marine Science, 67, 952-958.
Thomsen F, Laczny M & Piper W (2006) A recovery of harbor porpoises (Phocoena phocoena) in the southern North Sea? A case study off Eastern Frisia, Germany. Helgoland Marine Research, 60, 189–195.
Thomsen F, Laczny M & Piper W (2007) The harbour porpoise (Phocoena phocoena) in the central German Bight: phenology, abundance and distribution in 2002-2004. Helgoland Marine Research, 61, 283-289.
Tougaard S & Kinze CC (eds.) (1999) Proceedings from the Workshop: Sperm whale strandings in the North Sea. The event – the action – the aftermath. Rømø, Denmark 26-27 May 1998. Fisheries and Maritime Museum, Esbjerg, Biological Papers, 1, 1999.
Van Neer A, Jensen LF & Siebert U (2015) Grey seal (Halichoerus grypus) predation on harbour seals (Phoca vitulina) on the island of Helgoland, Germany. Journal of Sea Research, 97, 1-4.
Wiemann A, Andersen LW, Berggren P, Siebert U, Benke H, Teilmann J, Lockyer C, Pawliczka I, Skora K, Roos A, Lyrholm T, Paulus KB, Ketmaier V & Tiedemann R (2010). Mitochondrial Control Region and microsatellite analyses on harbour porpoise (Phocoena phocoena) unravel population differentiation in the Baltic Sea and adjacent waters. Conservation Genetics, 11, 195–211.
Wilkinson D & Worthy GAJ (1999) Marine mammal stranding networks. Pages 396-411 in J. R. Twiss and R. R. Reeves, eds. Conservation and management of marine mammals. Smithsonian Press, Washington DC.
This report should be cited as: Jensen L. F., Teilmann J., Galatius A., Pund R., Czeck R., Jess A., Siebert U., Körber P. & Brasseur S. (2017) Marine mammals. In: Wadden Sea Quality Status Report. Eds.: Kloepper S. et al., Common Wadden Sea Secretariat, Wilhelmshaven, Germany. Last updated 21.12.2017. Downloaded DD.MM.YYYY. qsr.waddensea-worldheritage.org/reports/marine-mammals-2017
All 2017 reports may be cited collectively as: Kloepper S., Baptist M. J., Bostelmann A., Busch J.A., Buschbaum C., Gutow L., Janssen G., Jensen K., Jørgensen H.P., de Jong F., Lüerßen G., Schwarzer K., Strempel R. & Thieltges D. (2017) Wadden Sea Quality Status Report. Common Wadden Sea Secretariat, Wilhelmshaven, Germany. Downloaded DD.MM.YYYY. qsr.waddensea-worldheritage.org
