
Photo: Martin Stock. Aerial photo of mudflats.
Subtidal habitats
K. Ricklefs, O. Franken, S. Glorius, F. Mascioli, P. Nielsen, H.-C. Reimers, A. Trampe
Published 2022
1. Introduction
This report deals with the status of subtidal habitats in the Wadden Sea. The term ‘habitat’ describes areas where ’physical, chemical and biological factors differ significantly from the surrounding environment‘ (Kostylev et al., 2001; Valentine et al., 2005). The adjective ‘subtidal’, on the other hand, describes the deeper parts of the Wadden Sea, which are more than 96% of the time submerged (Baptist et al., 2019). Using this threshold, 44% of the area of the trilateral Wadden Sea belongs to the subtidal (Figure 1).
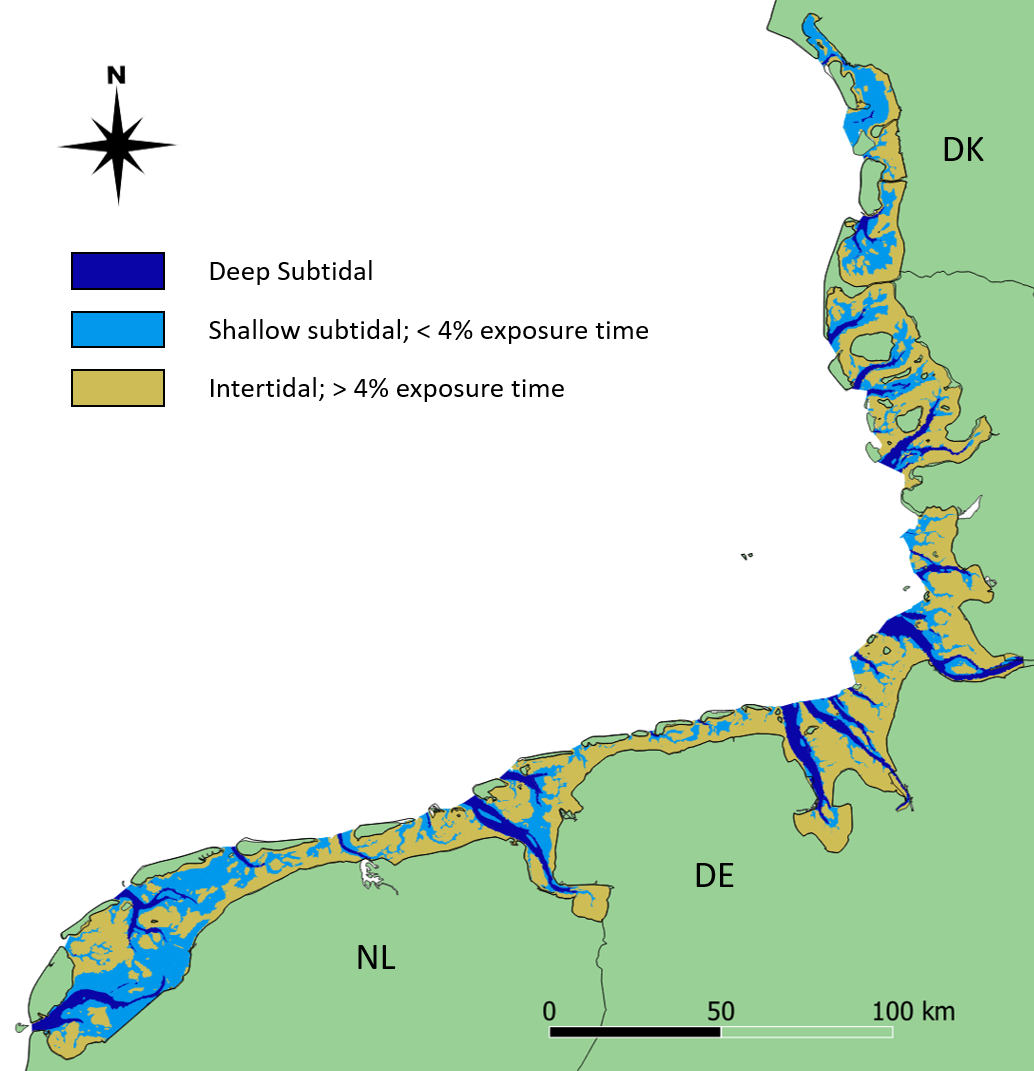 Figure 1: Distribution of deep and shallow subtidal (dark and light blue, respectively) and intertidal (yellow) areas in the trilateral Wadden Sea. The map is based on the ecotopes provided in Baptist et al. (2021).
Figure 1: Distribution of deep and shallow subtidal (dark and light blue, respectively) and intertidal (yellow) areas in the trilateral Wadden Sea. The map is based on the ecotopes provided in Baptist et al. (2021).
Subtidal benthic habitats are subject to a variety of natural and anthropogenic influences and stresses. For example, the natural redeposition of sediments can significantly alter a seabed area. Further, the OSPAR Commission identifies eutrophication and especially physical disturbances due to fishing with bottom contacting gears as major human impacts on seabed habitats. So, according to the OSPAR assessment (OSPAR Assessment Portal, 2017), 68% of the seafloor in the southern North Sea (this area includes the Wadden Sea) is disturbed by bottom contacting fishing (see also ICES, 2021).
For nature conservation, the European Union has introduced legislatives like the NATURA2000 Habitat Directive (HD), the Water Framework Directive (WFD), and the Marine Strategy Framework Directive (MSFD). Their implementation requires recurrent monitoring of the specific natural environments as well as an assessment of their ecological status. These obligations in conjunction with the demand for an ecosystem-based Wadden Sea management led to considerable progress in subtidal habitat mapping in the Wadden Sea. However, discrepancies in the interpretation of the EU legislation, as well as additional national guidelines, lead to different monitoring strategies in the three Wadden Sea countries. In the Netherlands and Denmark, for example, the focus of monitoring is on recording mussel stocks and other benthic species on the basis of discrete grid samples (see Annex). Whereas in Germany, sampling of sublittoral mussel stocks is carried out on a more random basis, while the focus of activities is more on a comprehensive mapping of sediments, sediment structures, habitats and biogenic and geogenic reef structures by means of hydroacoustic survey techniques and accompanying sampling (see Annex).
This report presents the actual status (2018-2021) of subtidal habitat mapping in Denmark, Germany and The Netherlands, considering the intention and the survey methods that were applied. For the identification and definition of habitats, the classification scheme of the European Nature Information System EUNIS (Davies et al., 2004; Evans et al., 2016) is used in this report. The relevant subtidal habitats of the Wadden Sea are listed under the EUNIS level A4 and A5, including rock and other hard substrates in highly turbid conditions, biogenic constructions like Sabellaria reefs or mussel beds as well as subtidal sediments ranging from boulders and cobbles to coarse, mixed and fine sands. For methods and data used in this report see Annex.
2. Status and Trends
2.1 Blue mussel beds (EUNIS marine habitat code A4.241/A5.625)
The results of the Dutch annual blue mussel (Mytilus edulis) stock assessment carried out since 1992 (see Annex) show that especially the relatively shallow and sheltered subtidal areas seaward of the ‘Afsluitdijk’ are most suitable for the survival of blue mussel beds over a longer period of time. More recent investigations further indicate that a relatively low salinity in those areas results in low starfish predation pressure (Troost et al. 2022.). The blue mussel occurrence in the western Dutch Wadden Sea is depicted in the map of Figure 2. Figure 3 shows the annual biomass (fresh weight) of blue mussels for the entire survey period since 1992. Over the last decade, larger quantities of mussel seed were found in the years 2013, 2017 and 2019. In the period of 2013-2019 the perennial mussel stock was relatively large with an average of 38 ±16 (stdv) M kg compared to the period of 2003-2012 with an average of 17.8 ±7.5 (stdv) M kg.
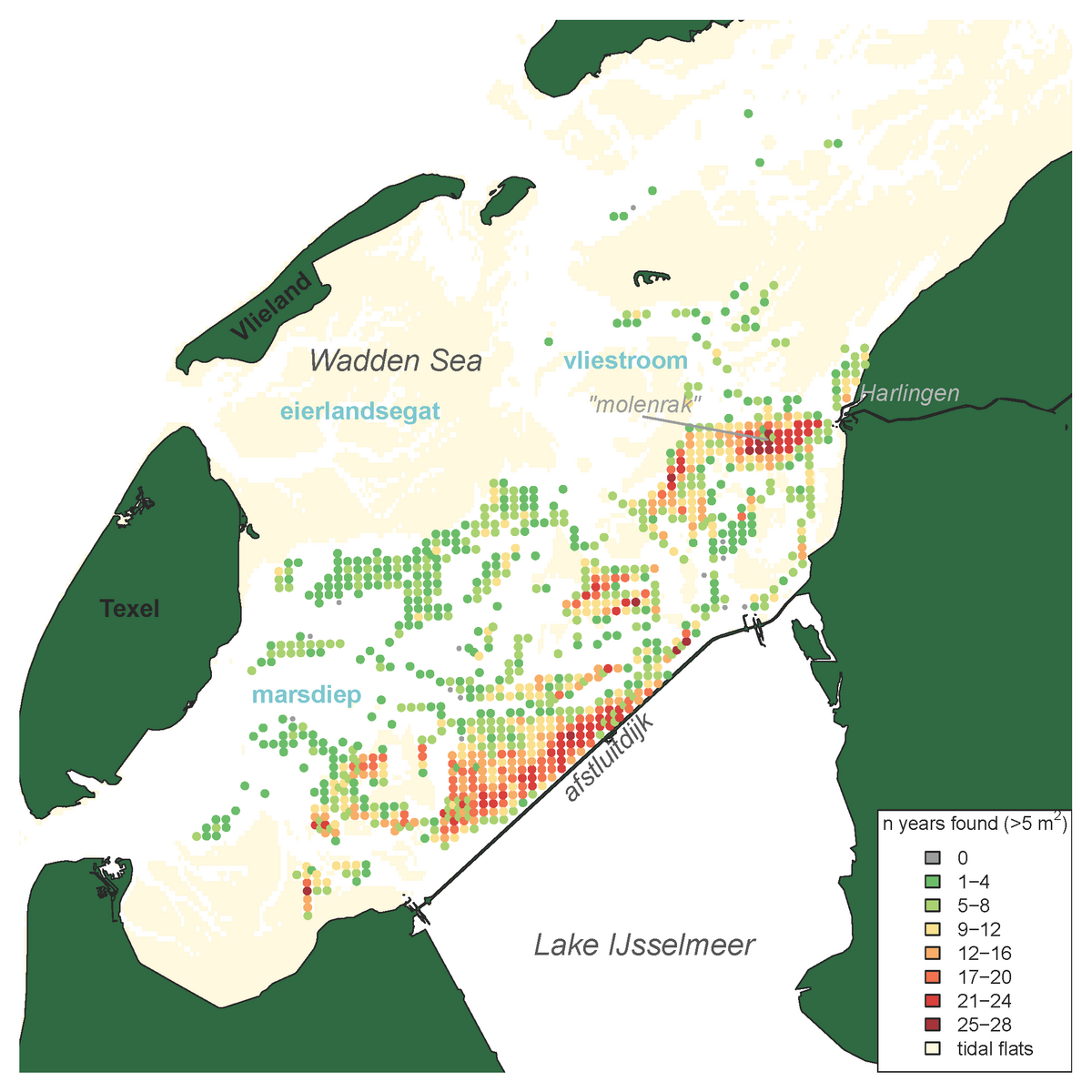 Figure 2: Distribution map of blue mussels (Mytilus edulis) in the subtidal areas of the tidal basins Marsdiep and Vliestroom (the smaller Eierlandsegat tidal basin is not monitored) indicating the frequency of occurrence per sampling station for stations with a mussel density >5 ind. m-2 and sampled more than four times, calculated for all years in which the species was recorded in the annual subtidal mussel stock assessment (1992-2019)
Figure 2: Distribution map of blue mussels (Mytilus edulis) in the subtidal areas of the tidal basins Marsdiep and Vliestroom (the smaller Eierlandsegat tidal basin is not monitored) indicating the frequency of occurrence per sampling station for stations with a mussel density >5 ind. m-2 and sampled more than four times, calculated for all years in which the species was recorded in the annual subtidal mussel stock assessment (1992-2019)
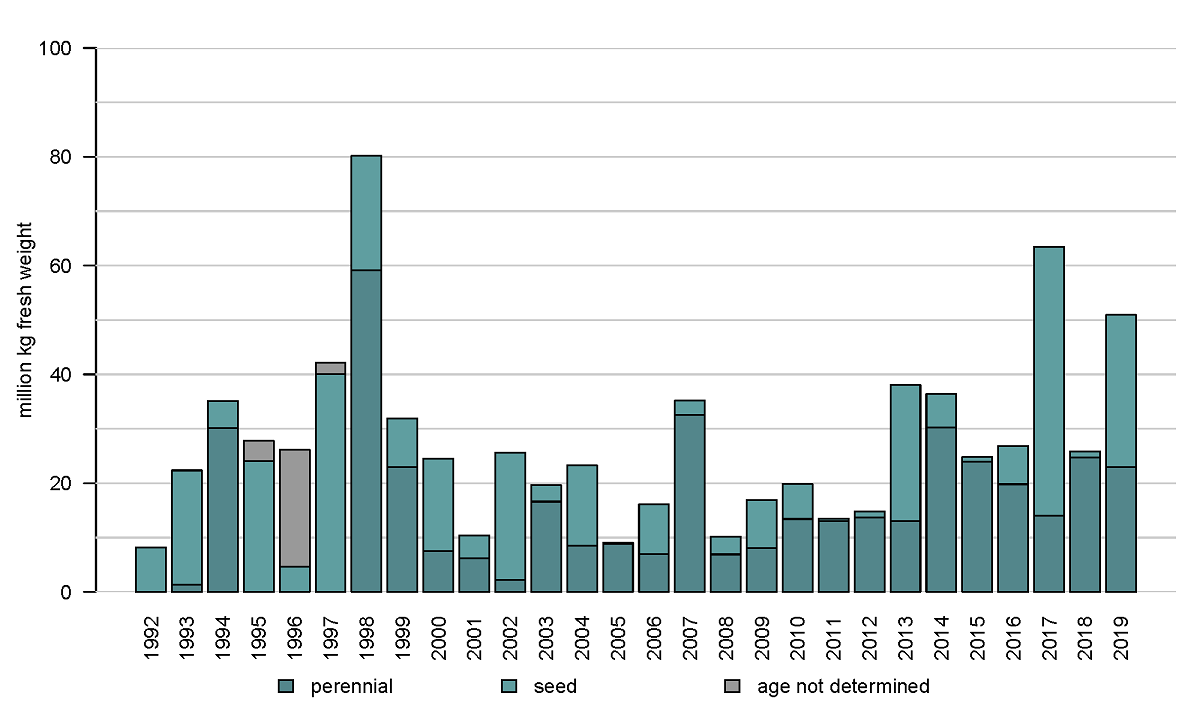 Figure 3: Annual biomass stock of the blue mussel (Mytilus edulis) in the subtidal areas of the tidal basins Marsdiep and Vliestroom.
Figure 3: Annual biomass stock of the blue mussel (Mytilus edulis) in the subtidal areas of the tidal basins Marsdiep and Vliestroom.
In the East Frisian Wadden Sea of Lower Saxony, several research and mapping programs focus on mussel beds features and detection methods in the intertidal areas (Folmer E. et al., 2017; Capperucci et al., 2020). Recent hydroacoustic investigations provide new data on both wild mussel beds as well as blue mussel culture plots in the subtidal areas. Most of the identified mussel beds are located in very shallow waters, close to the mean low-tide level. They are generally the continuation of intertidal mussel beds towards the subtidal, without a real differentiation between the two zones (Figure 4).
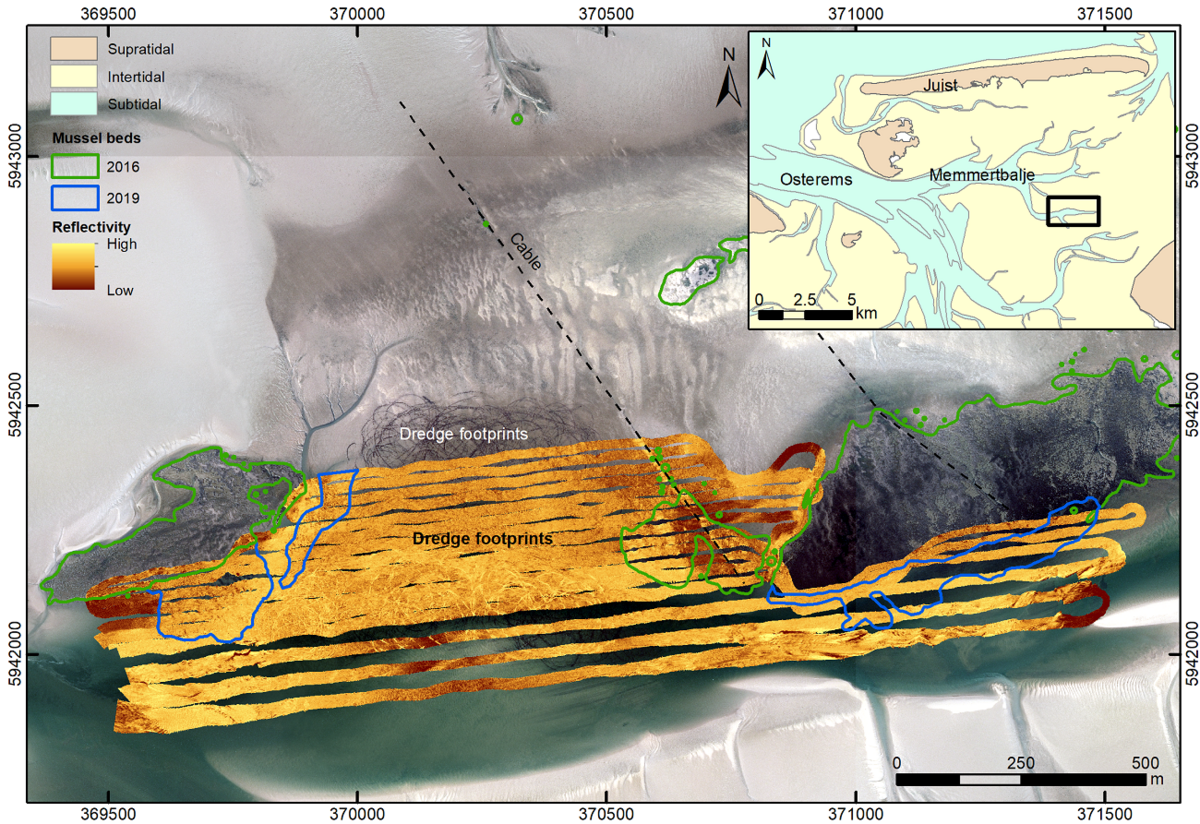 Figure 4: Culture plot (middle part) and adjacent wild blue mussel beds (marked by green and blue polygons) mapped based on aerial photographs (2016 – green polygons) and acoustic imaging (multibeam echosounder survey 2019 – blue polygons).
Figure 4: Culture plot (middle part) and adjacent wild blue mussel beds (marked by green and blue polygons) mapped based on aerial photographs (2016 – green polygons) and acoustic imaging (multibeam echosounder survey 2019 – blue polygons).
Almost area-wide sonar surveys in the German Wadden Sea of Schleswig-Holstein (see Annex) since 2009 indicate that at many sites, where blue mussels were discovered earlier by dredge-sampling (Ricklefs et al., 2020), the density of settlement was most likely relatively low or not stable over time. On the other hand, there is a good fit between sonar and sample data at those sites, where mussels were found recurrently. However, only 2 to 3 stable beds can be classified as biogenic reefs according to the guidelines of the EU Habitats Directive. All of them have no connection to intertidal beds and were found only in sheltered areas with very shallow water. Their spatial extent is quite stable over time (Figure 5). The multi-year blue mussel assemblages found at the sites are intensively associated with increasing populations of the invasive Pacific oyster (Magallana gigas) as well as the also invasive common slipper limpet (Crepidula fornicata).
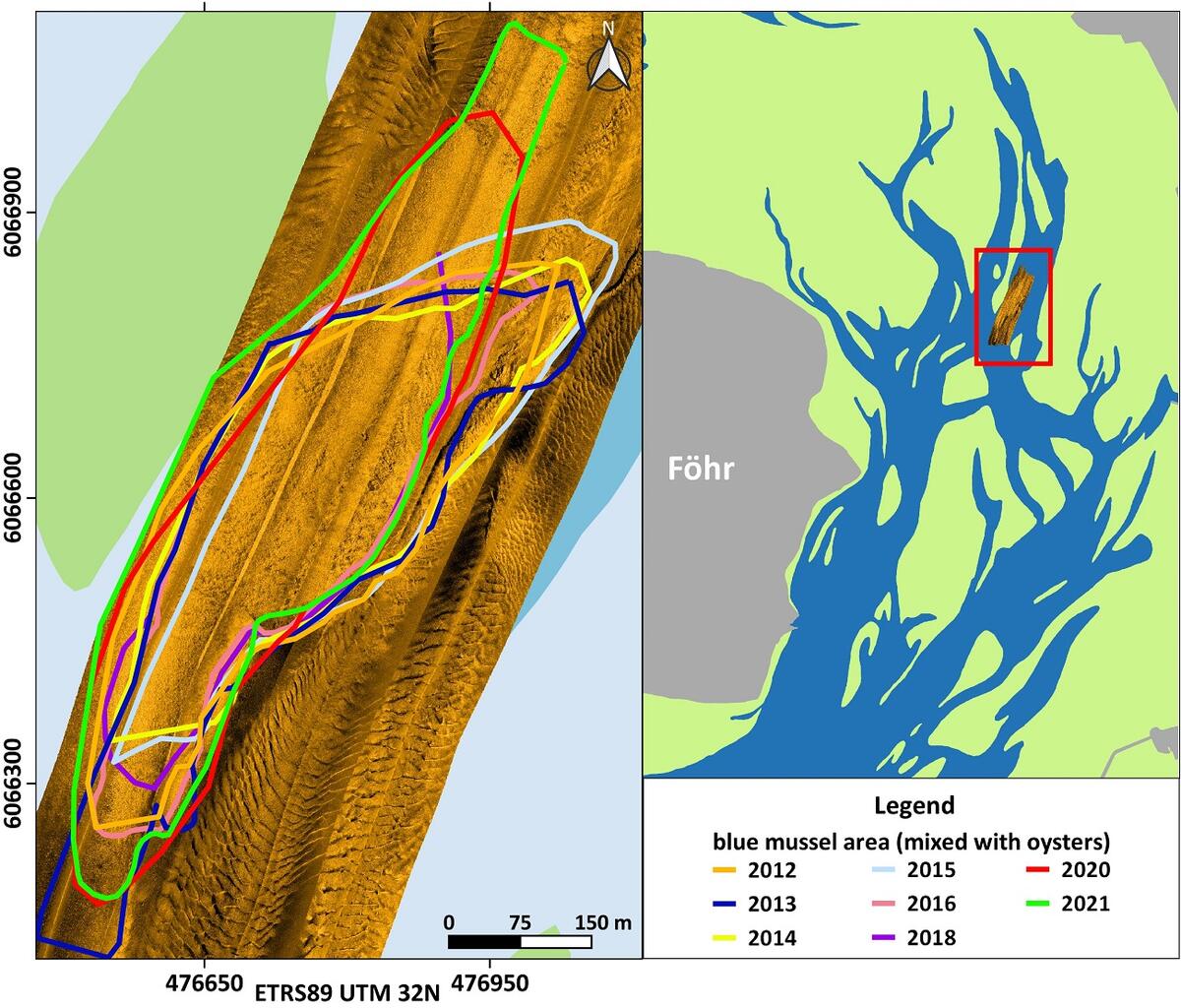 Figure 5: Side-scan sonar mosaic of a subtidal mussel reef in the vicinity of Föhr. The coloured lines mark the spatial extension of the habitat in different years.
Figure 5: Side-scan sonar mosaic of a subtidal mussel reef in the vicinity of Föhr. The coloured lines mark the spatial extension of the habitat in different years.
In 2017-2018, for the first time since 2008, a blue mussel and Pacific oyster stock assessment based on dredge samples was carried out in the Danish part of the Wadden Sea. In the subtidal, the stock survey included 58 randomized dredge sampling stations (Figure 6). At each station both species were assessed (details see Nielsen et al., 2019). Subtidal blue mussel beds are found only in the Ho Bugt and at the northern end of Fanø and in co-occurrence with Pacific oysters at all sites. It has not yet been investigated whether those mussel beds can be characterized as biogenic reefs according to the Danish definition of reefs (Dahl & Petersen, 2018).
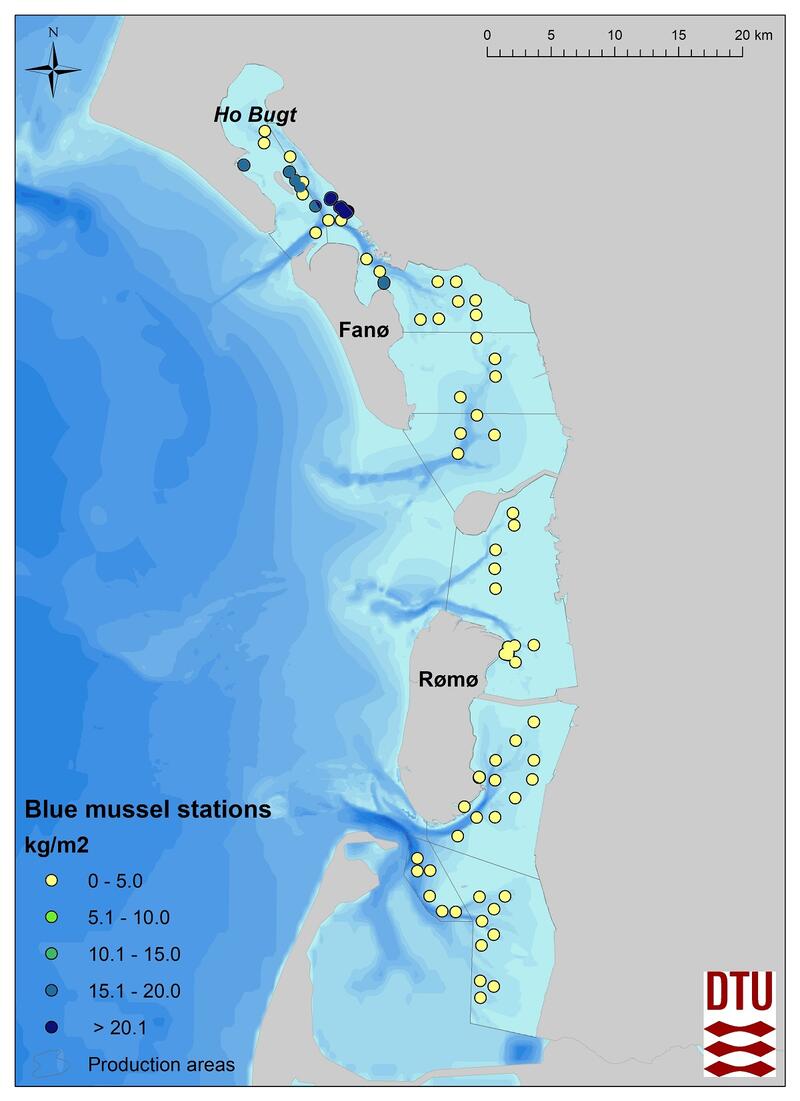
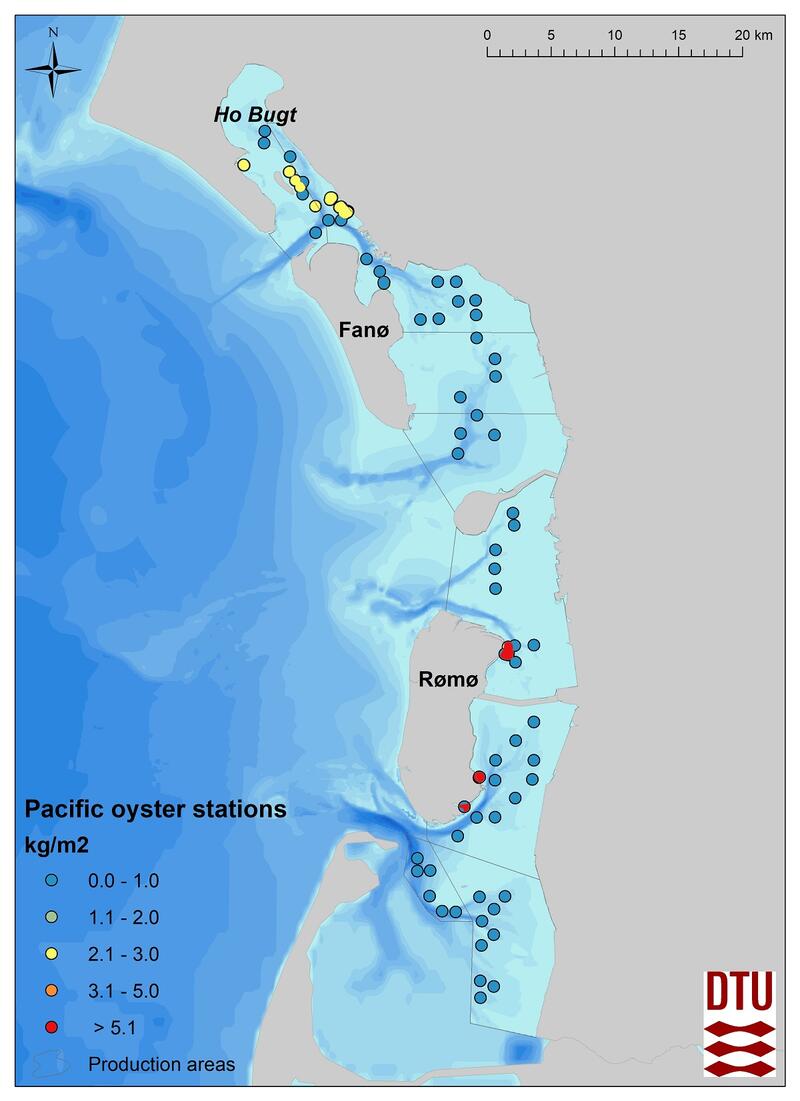 Figure 6: The distribution and densities (kg/m2) of blue mussels (Mytilus edulis) and Pacific oysters (Magallana gigas) in 2017-2018, Denmark.
Figure 6: The distribution and densities (kg/m2) of blue mussels (Mytilus edulis) and Pacific oysters (Magallana gigas) in 2017-2018, Denmark.
Based on the present state of knowledge presented so far, it can be stated that subtidal beds with perennial stocks of solely blue mussels still exist in the Netherlands and probably also in Lower Saxony. In Schleswig-Holstein and Denmark subtidal blue mussels always co-occur with oysters.
2.2 Pacific oyster beds (EUNIS marine habitat code A5.643)
The distribution area of Pacific oysters (Magallana gigas) in the Western Dutch Wadden Sea coincides to some extent with that of the blue mussels. However, the area close to the ‘afsluitdijk’ and the area ‘molenrak’ is not as important for Pacific oysters as it is for blue mussels (Figure 2 and 7). The reef-forming Pacific oyster was first present in the dataset in 2005 and showed a strong increase in 2008 (Figure 8). Due to successful oyster spatfall events (visible in the oyster density, not shown here) in the years 2011, 2014 and 2015 the oyster biomass was relatively high in the years 2012, and 2015 until 2017. In the period of 2017-2019 the number of small oysters in the survey data was very limited and the oyster biomass showed a strong decrease in 2018. In 2019 the biomass decreased even more to about half of the stock found in 2008.
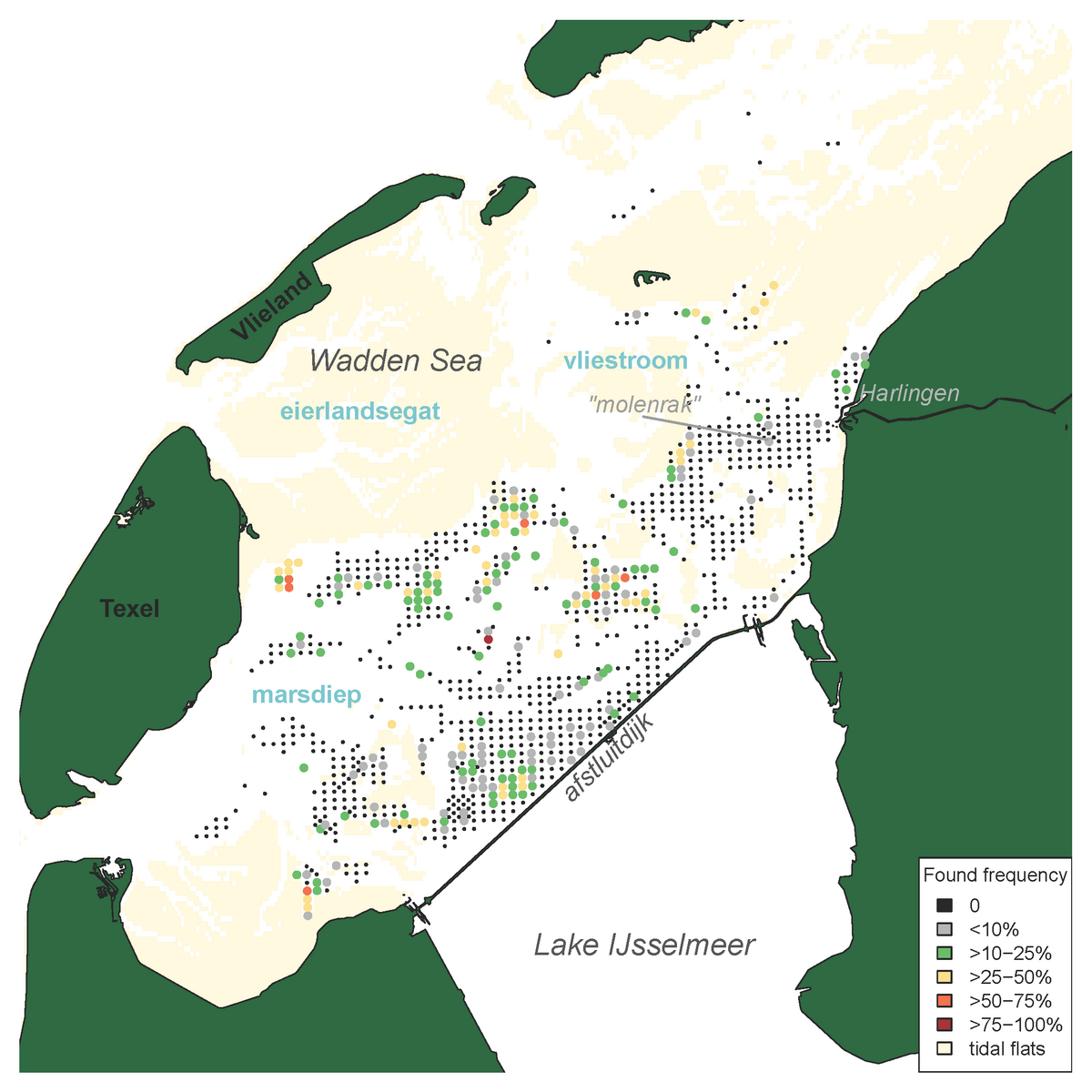 Figure 7: Distribution map of the Pacific oyster (Magallana gigas) in the subtidal areas of the tidal basins Marsdiep and Vliestroom (located in the western Dutch Wadden Sea) indicating the frequency of occurrence per sampling station for stations sampled more than four times, calculated for all years in which the species was recorded in the annual subtidal mussel stock assessment (2003-2019).
Figure 7: Distribution map of the Pacific oyster (Magallana gigas) in the subtidal areas of the tidal basins Marsdiep and Vliestroom (located in the western Dutch Wadden Sea) indicating the frequency of occurrence per sampling station for stations sampled more than four times, calculated for all years in which the species was recorded in the annual subtidal mussel stock assessment (2003-2019).
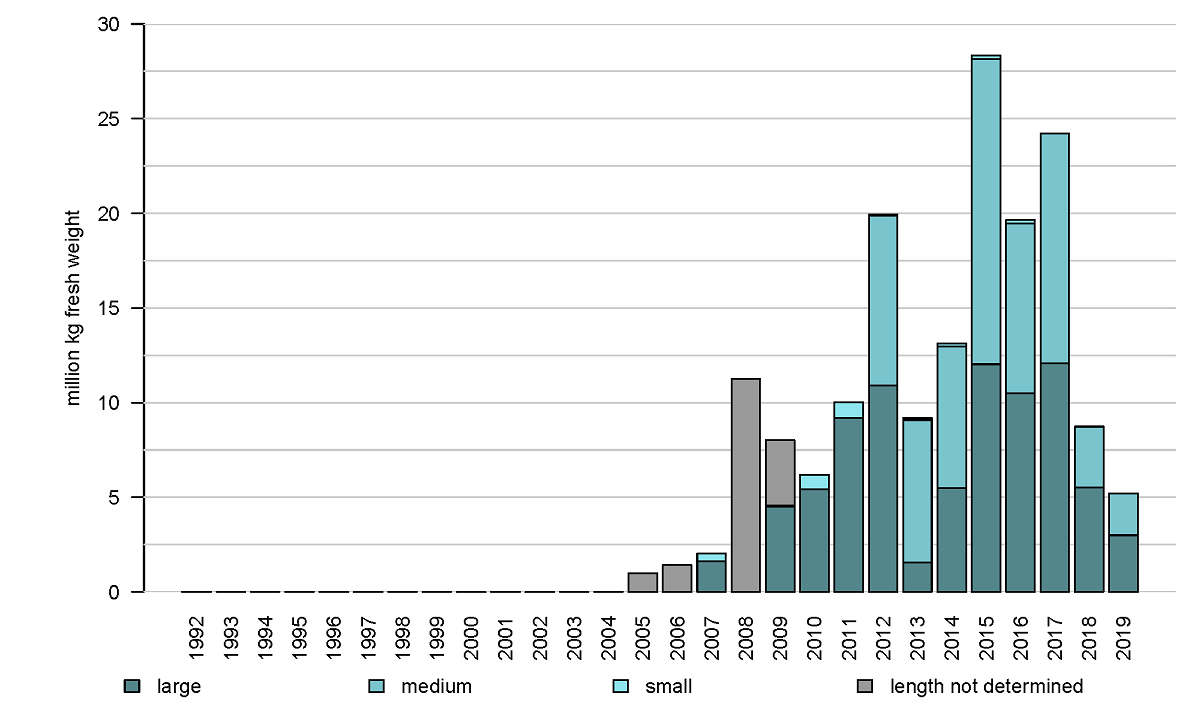 Figure 8: Annual biomass stock of the Pacific oyster (Magallana gigas) in monitored subtidal areas of the western Dutch Wadden Sea.
Figure 8: Annual biomass stock of the Pacific oyster (Magallana gigas) in monitored subtidal areas of the western Dutch Wadden Sea.
While for the Wadden Sea of Lower Saxony the knowledge about subtidal oyster occurrences is still rather limited it is quite obvious that in Schleswig-Holstein oysters, more and more become the dominant species of subtidal mussel beds. Besides mixed beds of blue mussels and oysters (“Oyssel beds”), a type of oyster bed was found in the northern part of the Wadden Sea of Schleswig-Holstein that was not described earlier for German waters. At these sites solely oysters are lying individually with their rounded side on or often up to ¾ sunken in fine sandy to sandy-muddy sediments (Figure 9). So, the presence of oysters here is not related to any kind of hard substrate and according to our present knowledge, blue mussels do not colonize these sites. Instead, the flat upper shell of the oyster functions as a hard substrate and settlement ground for a variety of other animals and plants. These pure oyster beds were found preferentially in the vicinity of but not necessarily directly connected to larger eulittoral oyster beds.
In the Danish Wadden Sea, the Pacific oyster was first observed in 1996 and was reported as increasing in annual stock assessments from 2005 to 2008 and has continued to increase in the 2017-2018 assessment. As noted above, oysters always co-occur with blue mussels. The spatial extent and structure of these subtidal mixed beds is still largely unknown.
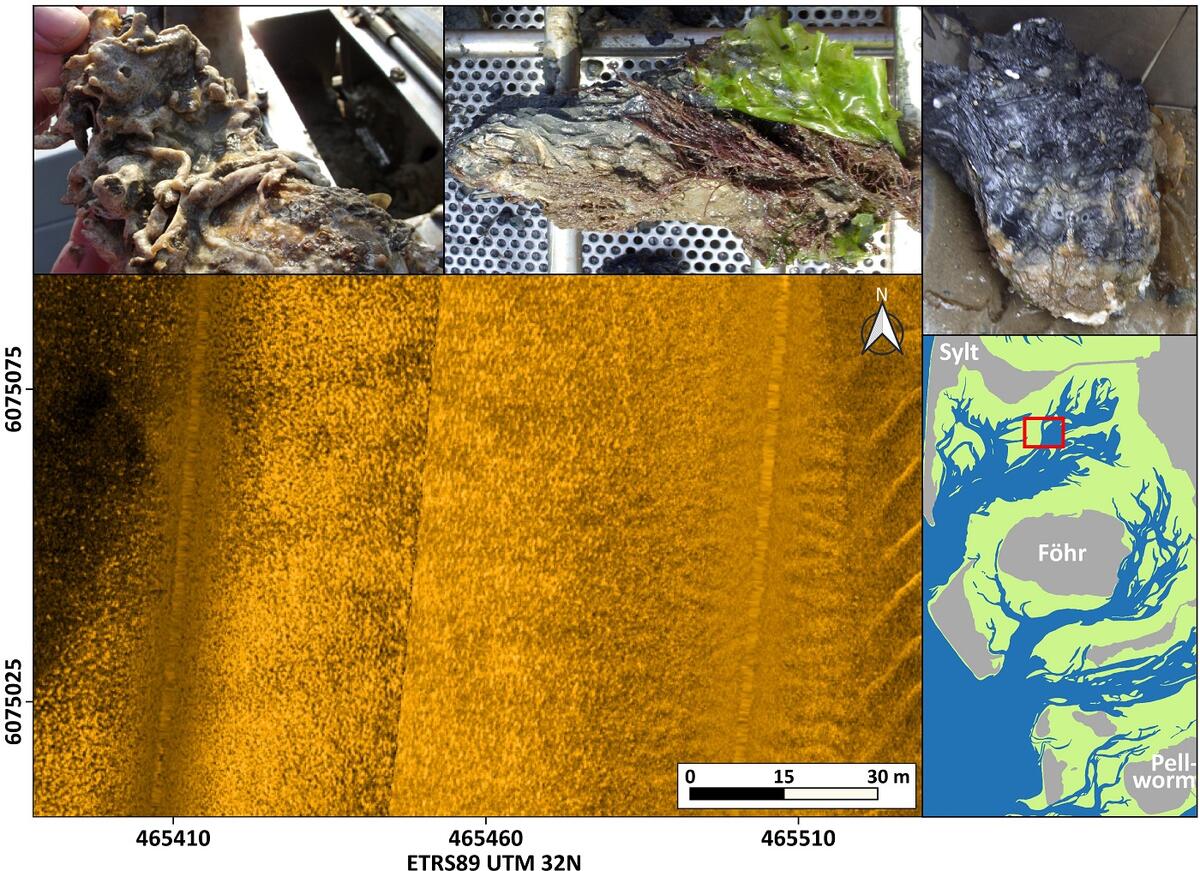 Figure 9: Side-scan sonar mosaic of a Pacific oyster (Magallana gigas) bed on fine sandy to muddy-sandy sediments. Numerous bright centers of acoustic backscattering are characteristic for such beds. The photographs depict how intensively the upper side of the oysters is settled by epibenthos and how deeply the lower, rounded side of the oysters can be sunken into the sediment (indicated by the dark color on the right photo).
Figure 9: Side-scan sonar mosaic of a Pacific oyster (Magallana gigas) bed on fine sandy to muddy-sandy sediments. Numerous bright centers of acoustic backscattering are characteristic for such beds. The photographs depict how intensively the upper side of the oysters is settled by epibenthos and how deeply the lower, rounded side of the oysters can be sunken into the sediment (indicated by the dark color on the right photo).
2.3 Sargassum muticum on shallow slightly tide-swept infralittoral mixed substrata (EUNIS marine habitat code A3.315)
Not far away from Pacific oyster beds on fine sandy to muddy sandy substrate as described above, some stocks of the also invasive algae Sargassum muticum were found in the northern part of back-barrier flats of the island of Sylt (Figure 10). Here, the algae settle in wider, shallow, and near-island subtidal areas with low current velocities. Individual specimens or stands have not been detected so far in other subtidal regions of the northern German Wadden Sea.
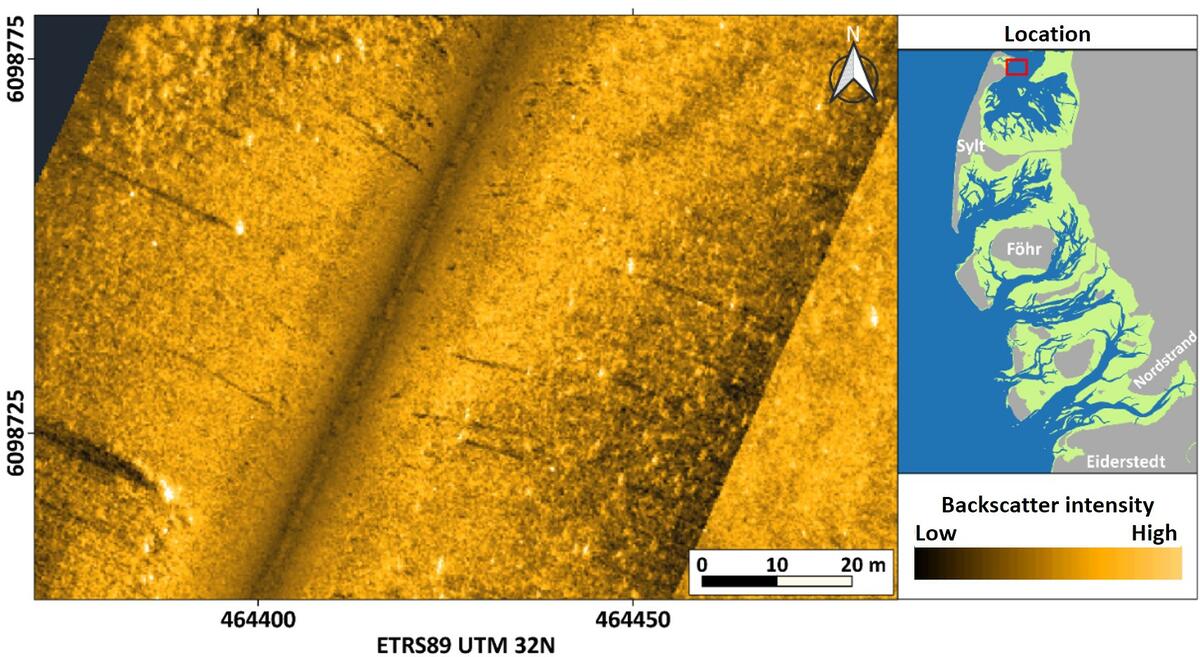 Figure 10: Side-scan sonar mosaic showing numerous algae of the species Sargassum muticum (Japanese wireweed) rising high from the seafloor - bright centres of high backscatter with dark shadows behind.
Figure 10: Side-scan sonar mosaic showing numerous algae of the species Sargassum muticum (Japanese wireweed) rising high from the seafloor - bright centres of high backscatter with dark shadows behind.
2.4 Lanice conchilega beds (EUNIS marine habitat code A5.137)
The sand mason worm (Lanice conchilega) is able to form large and dense patches, where individual worms construct tubes built from sand grains and shell fragments. These tubes can protrude several centimeters above the sediment, and thereby create a distinct habitat with altered currents and increased sedimentation. The tubes can serve as settlement substrate for other organisms (e.g., juvenile blue mussels). Lanice conchilega is, therefore, considered an important ecosystem engineer in the subtidal Wadden Sea.
In the Dutch subtidal Wadden Sea, a large sampling campaign was conducted within the Waddenmozaïek project (see Annex) in 2019, in which 1303 samples were collected within the study area. In 11% of these samples L. conchilega was present (Figure 11). Abundances of up to ~11.850 individuals per square meter were observed in one sample (the mean observed abundance was ~375 individuals per square meter in samples where L. conchilega was present).
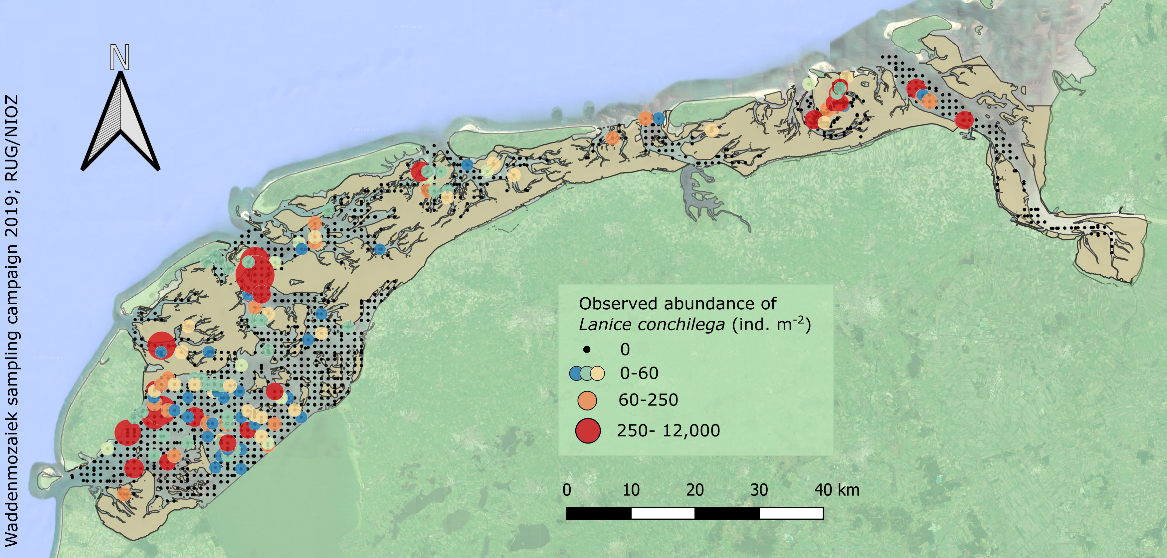 Figure 11: Sampled locations in the Dutch subtidal Wadden Sea in 2019 (black dots), with locations where Lanice conchilega was observed displayed as a gradient from small blue dots to larger red dots with increasing abundance.
Figure 11: Sampled locations in the Dutch subtidal Wadden Sea in 2019 (black dots), with locations where Lanice conchilega was observed displayed as a gradient from small blue dots to larger red dots with increasing abundance.
Where the abundance is high enough, the habitats of the worm can be detected quite reliably by hydroacoustic methods (Degraer et al., 2008; Heinrich et al., 2017). Using this approach, denser stocks of Lanice were mapped in the German Wadden Sea. Due to sand accumulation between the tubes, these beds can rise some centimeters up to one meter in height compared to the surrounding area (Vorberg et al., 2009). They are variably shaped and with spatial extents ranging from some square meters up to several hectares (Figure 12). In the northern part of the German Wadden Sea, the largest coherent sonar-detectable stocks do not occur in the Wadden Sea itself, but in the transition zone to the open North Sea (Figure 13).
Generally, the polychaete seems to tolerate a wide range of environmental conditions. Colonies were found in shallow sheltered waters, but also in deeper channels with high current velocities. The sediment distribution of the settling substrate ranges from very fine sand to gravely medium sand.
 Figure 12: a) Patches of Lanice conchilega in Norderney Riffgat, observed in the shallow sectors close to the low-tide line, as well as on the bottom of the main tidal channel. b) Acoustic image of a Lanice conchilega area, derived from multibeam echosounder backscatter intensity. c) Sample of Lanice conchilega on sandy sediments, collected by means of a van Veen grab.
Figure 12: a) Patches of Lanice conchilega in Norderney Riffgat, observed in the shallow sectors close to the low-tide line, as well as on the bottom of the main tidal channel. b) Acoustic image of a Lanice conchilega area, derived from multibeam echosounder backscatter intensity. c) Sample of Lanice conchilega on sandy sediments, collected by means of a van Veen grab.
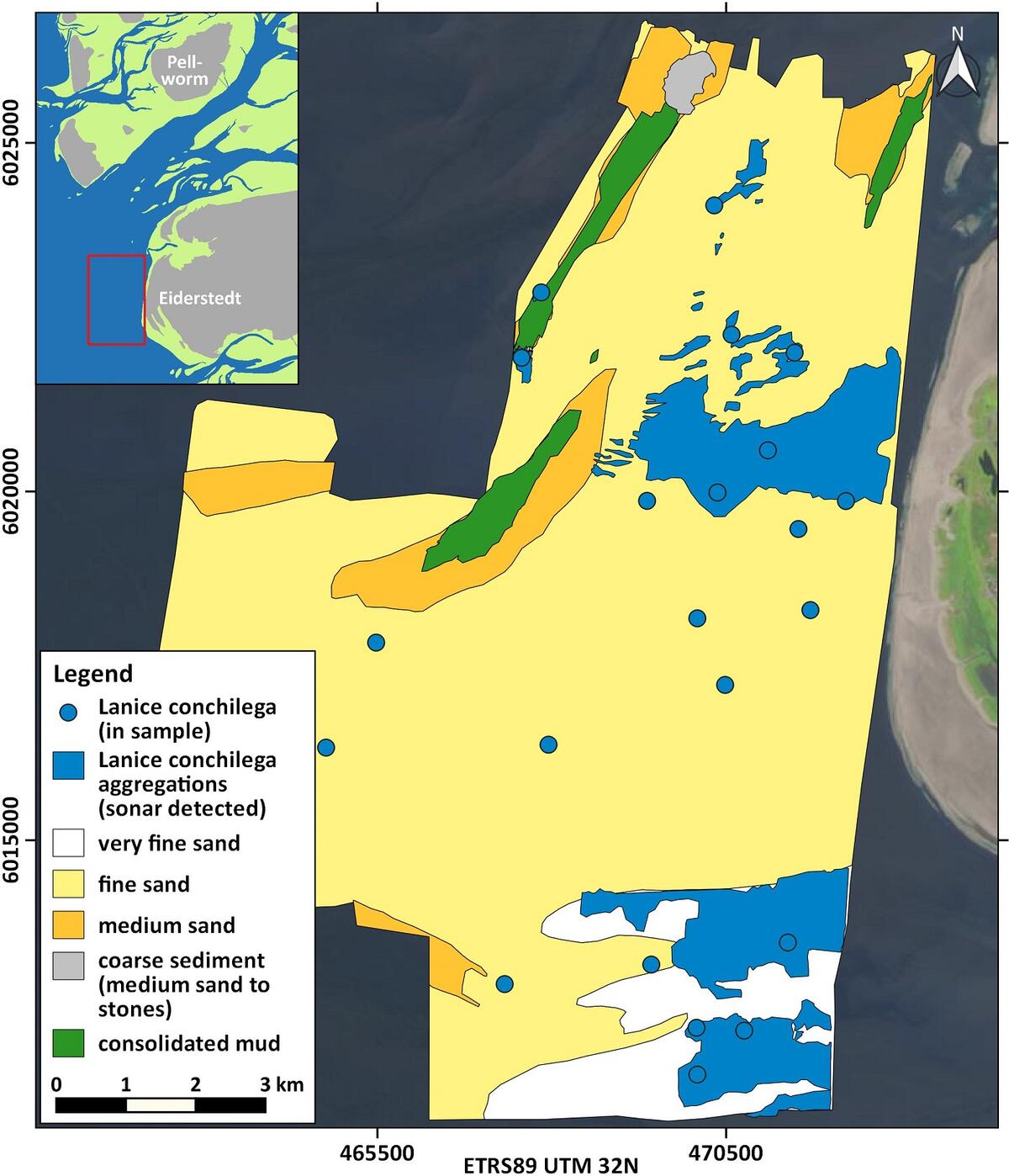 Figure 13: Distribution of Lanice conchilega in 2016 west of the Eiderstedt peninsula and in the outermost Eider estuary (blue dots: L. conchilega in grab samples and blue areas: L. conchilega aggregations detected by sonar).
Figure 13: Distribution of Lanice conchilega in 2016 west of the Eiderstedt peninsula and in the outermost Eider estuary (blue dots: L. conchilega in grab samples and blue areas: L. conchilega aggregations detected by sonar).
2.5 Coarse, medium and fine sand (EUNIS marine habitat code A5.2)
It was already stated in the previous QSR (Vorberg et al., 2017) that sandy sediments are representing by far the most common habitat in the Wadden Sea. Fine, medium, and coarse sands with less than 10 % of fines (< 0.063 mm) are very widespread in the deeper tidal channels and tidal inlets. The surface of these sands is often structured by bedforms of different size and shape (Figure 14), all indicating an ongoing redeposition of the substrate. Due to this high dynamic turnover, “classic” epibenthos like blue mussels is almost completely missing on these sediments. While the meiobenthic infauna shows a diverse species composition even in coarse sands, the number of macrobenthic infauna species is generally limited. Current investigations found indications that this niche is used quite intensively by the Atlantic jack knife clam (Ensis leei) (Schwemmer et al., 2019). In this way, E. leei has at least partially taken over the role of the cut trough shell (Spisula subtruncata), which was the most abundant bivalve species from the early 1980s (Tulp et al., 2010) in this type of unstable benthic habitat.
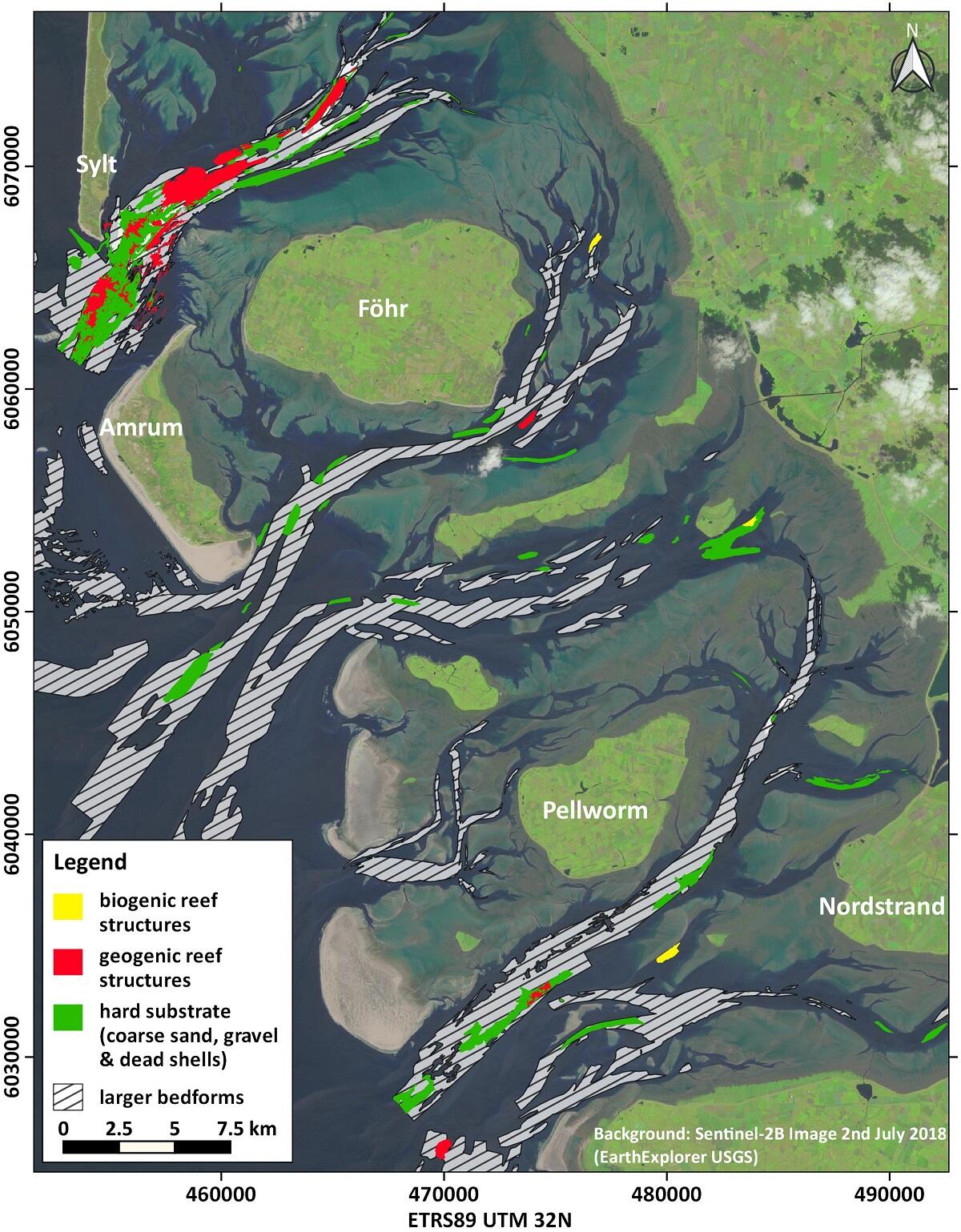 Figure 14: Distribution map of larger bedforms (wavelengths > 5 m), geogenic and biogenic reef structures, as well as hard substrates in the North Frisian Wadden Sea.
Figure 14: Distribution map of larger bedforms (wavelengths > 5 m), geogenic and biogenic reef structures, as well as hard substrates in the North Frisian Wadden Sea.
Figure 15 provides an overview of the composition of various sediment samples taken from the subtidal of the entire East Frisian Wadden Sea. The graph gives an indication of the dominance of sandy subtidal environments, which is also typical for other parts of the trilateral Wadden Sea. However, it also shows that in addition to pure sands, also habitats with a higher proportion of mud in the settlement substrate exist.
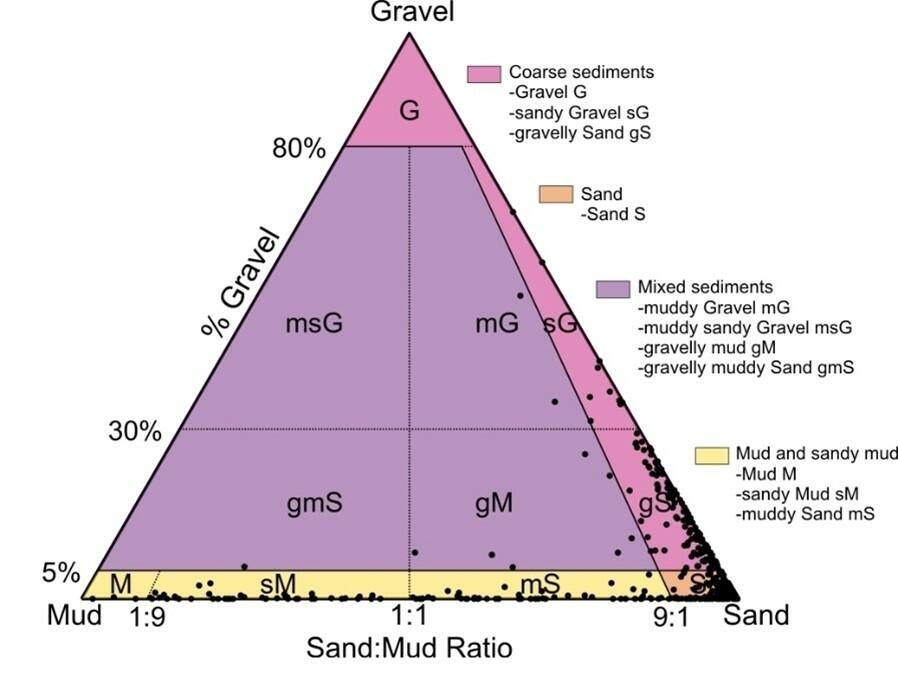 Figure 15: Sediment types in the subtidal of the East Frisian Wadden Sea, classified according to Folk (1954). Mud (< 0.063 mm), Sand (0.063 – 2 mm), Gravel (> 2 mm). Sample areas see Figure 23.
Figure 15: Sediment types in the subtidal of the East Frisian Wadden Sea, classified according to Folk (1954). Mud (< 0.063 mm), Sand (0.063 – 2 mm), Gravel (> 2 mm). Sample areas see Figure 23.
2.6 Muddy sand (EUNIS marine habitat code A5.241)
The EUNIS marine habitat code A5.241 is classified as the ‘sheltered lower shore and shallow sublittoral sediments of sand or muddy fine sand in fully marine conditions, which support populations of the urchin (Echinocardium cordatum) and the razor shell (Ensis siliqua or Ensis ensis)’. Here, we extend this definition to include, and focus on, the invasive Atlantic jack knife clam (Ensis leei) as its increase in distribution and abundance since its first record in the German Wadden Sea in 1981 (Essink, 1985) is noteworthy for the ecology of the subtidal Wadden Sea.
From 1992 onwards, an annual subtidal mussel stock assessment is carried out in the western Dutch Wadden Sea, which was expanded in 2015 to include areas of importance for the clam (Troost et al., 2021). This assessment clearly shows the emergence of the Atlantic jack knife clam (Figure 16). Apart from the peaks in 2005, 2011 and 2013, the stock size is more or less stable in the period of 2002-2016. In 2017 and 2019 large quantities of small clams were present in the samples resulting in an increase in biomass in following years. Over time, the fresh weight biomass increased in the western Dutch Wadden Sea from ~100 M kg in the period of 2015-2017, to 200 M kg in 2018 and 425 M kg in 2019. The Atlantic jack knife clam may therefore have a major impact on the carrying capacity of the Wadden Sea due to its extensive stock and filtration capacity.
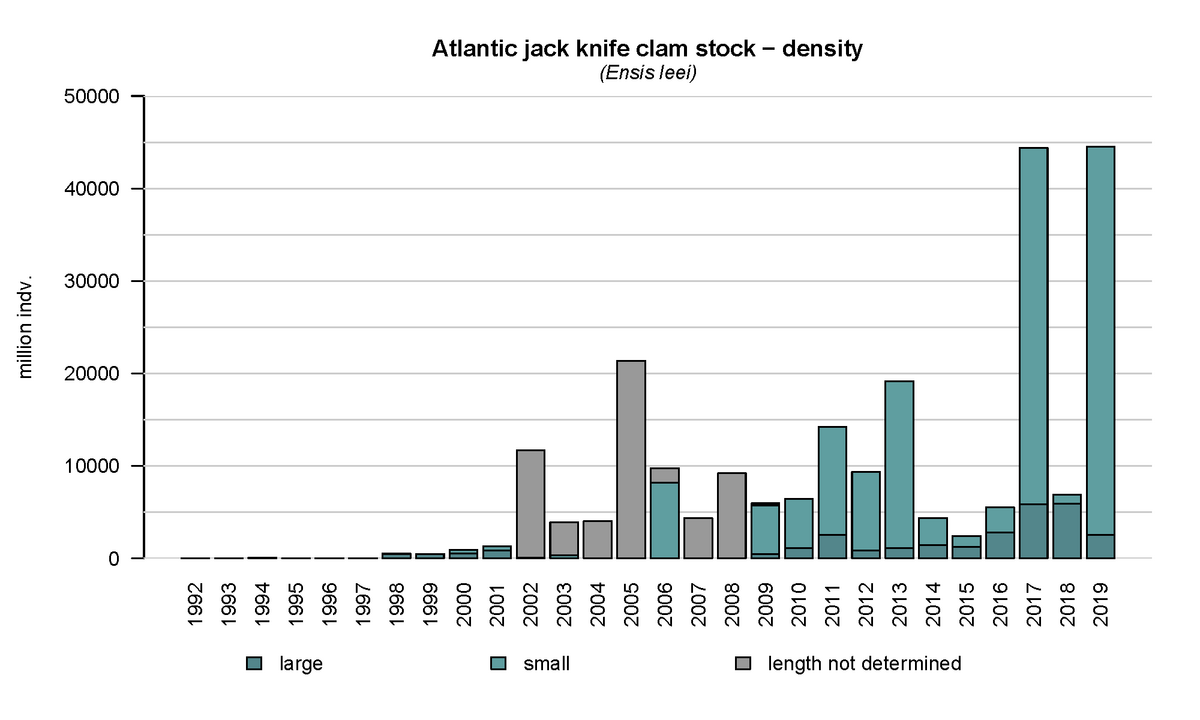 Figure 16: Annual density of the Atlantic jack knife clam (Ensis leei) in the subtidal areas of the tidal basins Marsdiep and Vliestroom located in the western Dutch Wadden Sea using data from the annual subtidal mussel stock assessment.
Figure 16: Annual density of the Atlantic jack knife clam (Ensis leei) in the subtidal areas of the tidal basins Marsdiep and Vliestroom located in the western Dutch Wadden Sea using data from the annual subtidal mussel stock assessment.
The current distribution of the Atlantic jack knife clam spans the whole subtidal Dutch Wadden Sea. In a sampling campaign in 2019 (Holthuijsen et al., 2019), the species occurred in all Dutch tidal basins, and was found in 36% of the samples (Figure 17), making it the most frequently encountered bivalve and the fifth most frequently encountered species overall. The species can be locally very abundant, for example, in the German transition zone to the North Sea, west of the Eiderstedt peninsula, where juveniles were observed in abundances up to 80.000 ind. m-2 (pers. communication Dr. P. Schwemmer, FTZ Büsum).
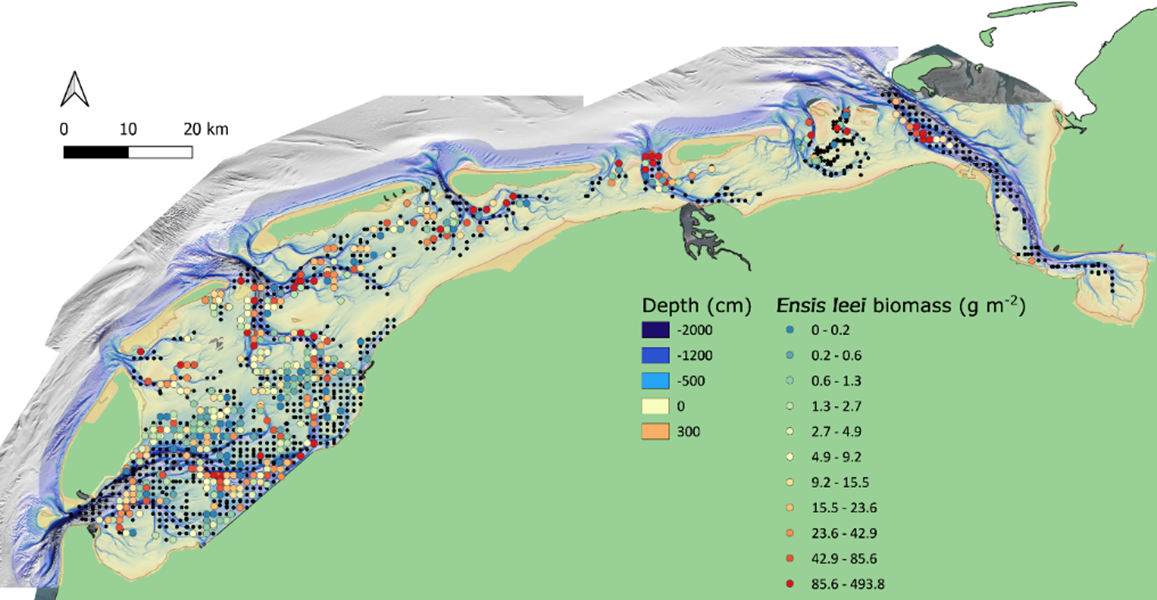 Figure 17: Sampled locations of the Waddenmozaïek project in the Dutch subtidal Wadden Sea in 2019 (black dots), with locations where the Atlantic jack knife clam (Ensis leei) was observed displayed as a gradient from blue dots to red dots with increasing biomass (grams m-2 of ash free dry weight).
Figure 17: Sampled locations of the Waddenmozaïek project in the Dutch subtidal Wadden Sea in 2019 (black dots), with locations where the Atlantic jack knife clam (Ensis leei) was observed displayed as a gradient from blue dots to red dots with increasing biomass (grams m-2 of ash free dry weight).
There seem to be areas where the occurrence of the Atlantic jack knife clam is relatively stable over time. Especially areas that are slightly deeper and highly dynamic appear to be hotspots for the clam (Figure 18). This pattern e.g., is consistent with their frequent presence in gullies near the island group ‘Rottum’ located in the eastern Dutch Wadden Sea (Glorius & Meijboom, 2020).
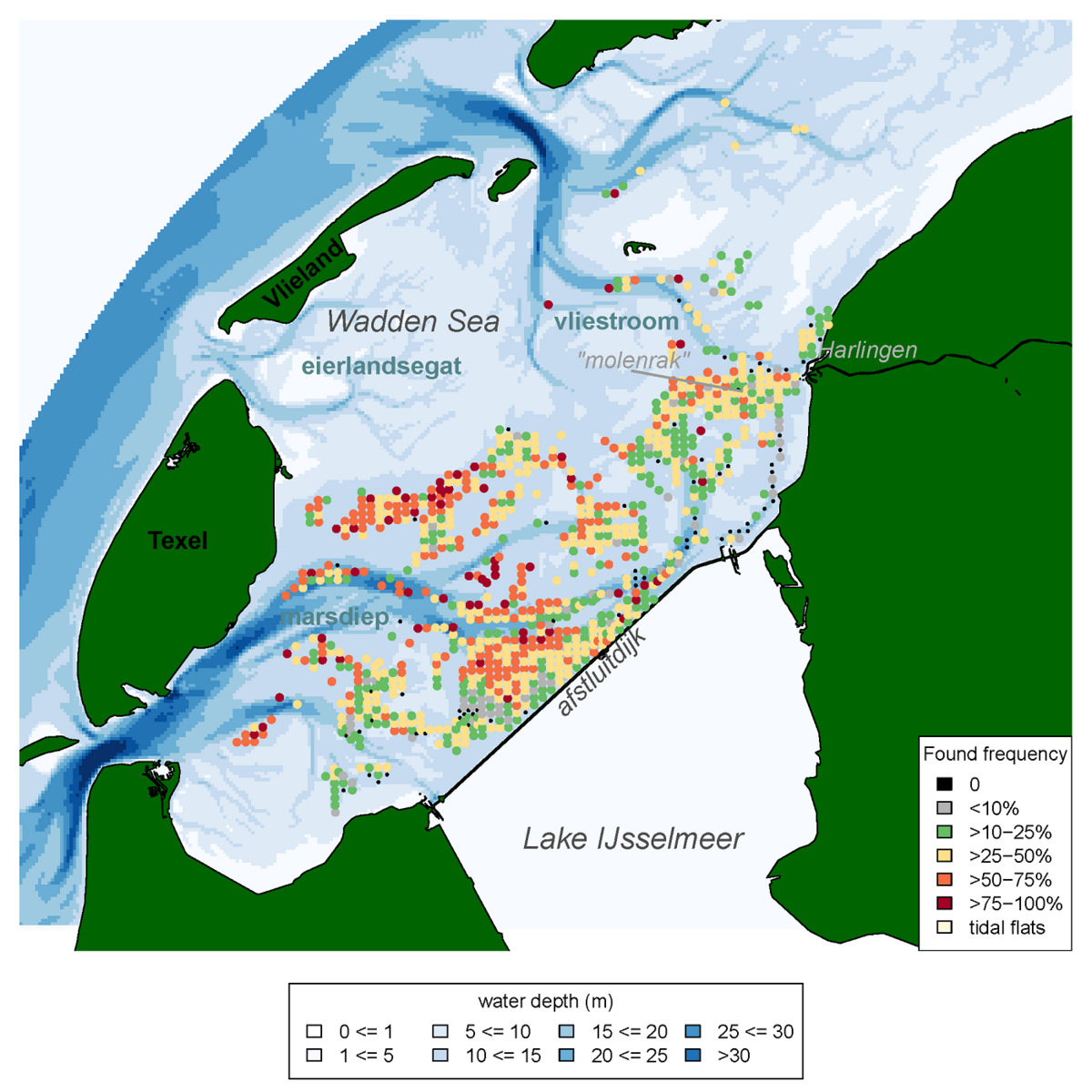 Figure 18: Frequency of occurrence of the Atlantic jack knife clam (Ensis leei) in the Western Wadden Sea, calculated for all years in which the species was recorded in the annual subtidal mussel stock assessment (1992-2019) that were sampled more than four times, and had a shellfish density >5 ind. m-2.
Figure 18: Frequency of occurrence of the Atlantic jack knife clam (Ensis leei) in the Western Wadden Sea, calculated for all years in which the species was recorded in the annual subtidal mussel stock assessment (1992-2019) that were sampled more than four times, and had a shellfish density >5 ind. m-2.
2.7 Sublittoral mud (EUNIS marine habitat code A5.3)
Although the seafloor in most tidal channels is mainly composed of sands, some areas occur where the channel bottom is dominated by muddy sediments. This is often the case for the innermost little tidal creeks, but also for certain areas in deeper major channels of a tidal basin. The high content of fine-grained material can be due to either special hydrodynamic conditions and/or the geological subsoil. Characteristic inhabitants of such muddy zones are brittle stars (Ophiuroidea). Also common on this kind of seafloor are tracks of beam trawling for brown shrimp (Crangon crangon). These partly deep grooves (Figure 19) can be very stable over a long period of time and, therefore, represent a severe human disturbance to soft sediment habitats. On sandy sediments, on the other hand, the traces are weak and are leveled out again by currents after a short time (Figure 20).
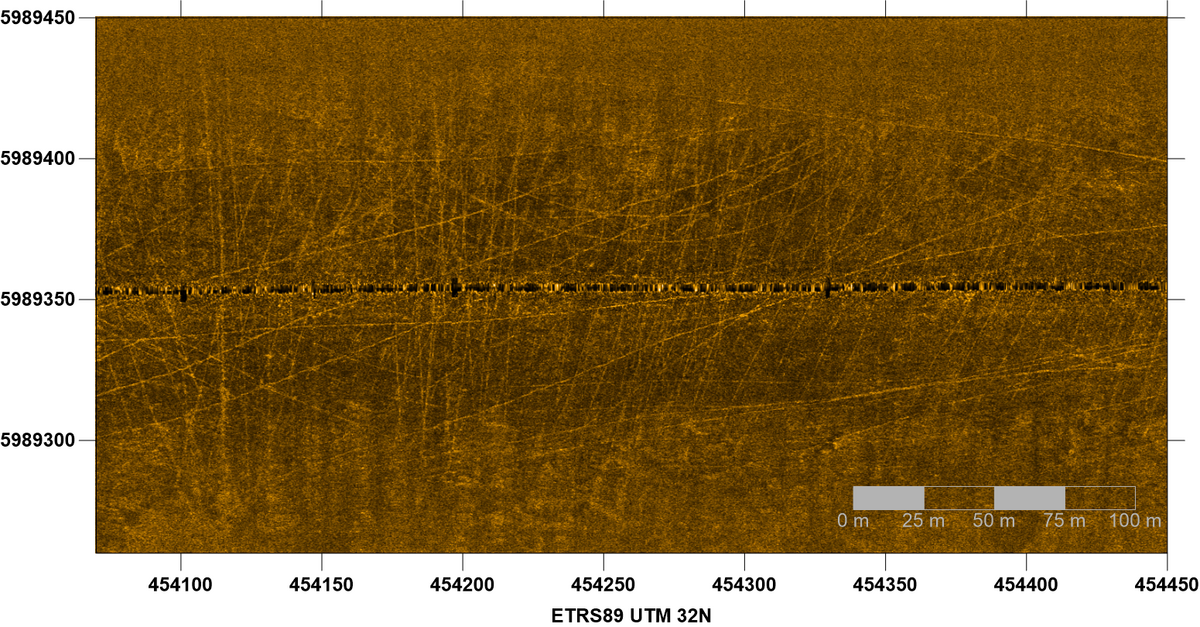 Figure 19: Side-scan sonar image of a muddy seabed in the outermost Norderelbe tidal channel showing numerous beam trawl tracks. Sonar data courtesy Dr. K. Schwarzer IfG Univ. Kiel.
Figure 19: Side-scan sonar image of a muddy seabed in the outermost Norderelbe tidal channel showing numerous beam trawl tracks. Sonar data courtesy Dr. K. Schwarzer IfG Univ. Kiel.

Figure 20: Side-scan sonar image showing shrimp nets and their traces on a sandy sea floor.
2.8 Circalittoral rock and other hard substrata (EUNIS marine habitat code A4)
Knowledge of biogenic and geogenic reef structures that meet the requirements of the Interpretative Manual of the EU Habitats Directive (EC, 2013) is quite variable in different parts of the trilateral Wadden Sea. This different level of information is partly due to how the member states categorize their part of the Wadden Sea within the legal framework of the EU Habitats Directive. Probably the best overview of reef structures is available for the Wadden Sea of Schleswig-Holstein (Figure 14). Here it can be seen that the number of sublittoral biogenic reefs is overall very limited (see chapter 2.1). Due to a certain natural similarity of the Wadden Sea areas, which still differ for their geological background, it can be assumed that this finding applies in a similar way to the Eastern Dutch, the Lower Saxon and the Danish Wadden Sea. The high numbers of blue mussels persistently found in the western Dutch Wadden Sea suggest that biogenic reef structures in the sense of the EU Habitats Directive should be present there. However, corresponding research results are not yet available.
The most famous and undoubted example of a biogenic reef structure in the subtidal of the Wadden Sea is that of the polychaete Sabellaria spinulosa. While fragments are repeatedly found in samples, the last proofs of Sabellaria reefs date back to the early 1990s (Vorberg, 1995, 2005).
Comprehensive knowledge of geogenic reef structures and hardgrounds is so far mainly available for the Wadden Sea of Schleswig-Holstein (Figure 14). Here, areas with larger numbers of bigger stones (> 20 cm) and boulders on the seabed are considered as geogenic reef structures. Subtidal deposits with a high content of gravel and/or dead shells are classified as hardgrounds. Often both environments overlap, but in any case, hardground environments can be considered as areas with a high biodiversity. For example, up to now 230 benthic species from 16 different major groups were identified in the Hörnumtief tidal inlet south of Sylt (Figure 14). 31 of these are red list species, 17 neobiota and 99 species, or 43% respectively, are obligatorily bound to hard substrates (Schückel et al. submitted). Recently, even European lobsters (Homarus gammarus) were found on a subtidal hard substrate site in the North Frisian Wadden Sea (personal comment by Dr. U. Schückel, NPA S.-H.)
Other special habitat types common in the subtidal of the Wadden Sea are consolidated clayey sediments and peat layers. The outcrops of these deposits are characterized by sharp edges, escarpments or plateaus (Figure 21). The soft rock often shows the burrows of piddock clams (Petricola pholadiformis, Figure 22) and/or forms the settling substrate for Anthozoa.
 Figure 21: Side-scan sonar mosaic of complex outcrop structures of consolidated fine-grained sediments partly covered by thin sand layers.
Figure 21: Side-scan sonar mosaic of complex outcrop structures of consolidated fine-grained sediments partly covered by thin sand layers.
 Figure 22: Sample of cohesive-consolidated sediment rich in plant remnants showing several piddock clam burrows.
Figure 22: Sample of cohesive-consolidated sediment rich in plant remnants showing several piddock clam burrows.
3. Assessment
Targets of the Wadden Sea Plan with respect to subtidal habitats are:
- A natural dynamic situation;
- An increased area of geomorphologically and biologically undisturbed subtidal areas;
- An increased area of, and a more natural distribution and development of natural mussel beds and Sabellaria reefs
It is a well-known fact that the Wadden Sea, which is mainly built up of soft mobile sediments and flowed through by strong tidal currents, is an exceptionally dynamic ocean realm. The living beings of this environment must therefore be capable to adapt to these hydrodynamic and morphodynamic conditions. Furthermore, seasonal and climatic changes naturally determine the living world in the Wadden Sea. The previous sections illustrate some examples of the dynamics of sublittoral habitats. So, for example blue mussel beds show a certain site stability, but the existing biomass of mussels varies strongly. Similar holds for the stocks of Pacific oysters. The increasing number of invasive species like the Pacific oyster (see thematic report on Alien Species) in a certain sense can be considered as a further dynamic impact on the Wadden Sea. It was found that pure sublittoral mussel beds no longer occur north of the Elbe River. Only mixed beds of mussels and oysters still exist here (Oyssel beds). Furthermore, there are indications that in these beds the number of blue mussels is decreasing while that of oysters is increasing and oysters are increasingly also colonising seafloor areas without any hard substrate. Hard substrates on the other hand, at least in the North Frisian Wadden Sea, are increasingly formed by the invasive slipper limpet (Crepidula fornicata).
Although not explicitly quantified, it can be assumed that human activities in the Wadden Sea, such as bottom-contacting fishing, dredging and coastal protection measures, can lead to habitat destruction and thus alter natural dynamics. However, to our knowledge, the extent of such impacts has not changed significantly since the last QSR. On the other hand, first measures have been taken in the Netherlands to exclude shrimp- and mussel seed fisheries in parts of the Wadden Sea and in 2021 these areas were further extended. Since 2017 some more areas in the Schleswig-Holstein Wadden Sea have come closer to the goal of an increase of geomorphologically and biologically undisturbed subtidal areas, as mussel fishing was prohibited. However, shrimp fishing is still allowed. That means that the third Wadden Sea Plan goal of an increased area of, and a more natural distribution and development of natural mussel beds and Sabellaria reefs is still quite far from being reached.
In this context, we consider the last-mentioned objective of the Wadden Sea Plan to be a bit too narrowly formulated. The terms ‘mussel beds’ and ‘Sabellaria reefs’ could be generalized by using ‘biogenic reef structures’. Furthermore, geogenic reefs should also be included, since they provide important habitats with a high biodiversity.
4. Recommendations
4.1 Recommendations for management
-
Establishing a commonly accepted understanding and definition of the different habitats that occur in the Wadden Sea, including a comprehensive list of relevant habitats in terms of scientific interest and and conservation value;
-
Review of targets: Instead of general and qualitative statements, as formulated in the Wadden Sea Plan, revised targets should meet the requirements of the Marine Strategy Framework Directive of measurable targets and associated indicators that allow for monitoring and assessment;
-
Support the formation of a trilateral expert group on subtidal habitat mapping;
-
Develop possible management scenarios to reduce already known (e.g. bottom-contacting fishing) but not necessarily already quantified human pressures and impacts on the seabed and its habitats (ICES, 2021).
4.2 Recommendations for monitoring and research
Harmonization of internationally established state-of-art methods applied for habitat mapping, including:
-
Implementation of structural, long-term survey campaigns over large spatial extents to track changes in sediment and benthic community composition in time and space.
-
Development of an integrated approach for hydroacoustic survey and ground truthing techniques.
-
Unifying sampling methodology and gear to detect species that play a key role in Wadden Sea ecology, e.g. habitat forming species.
-
Regular workshops for joint analyses and assessment of data.
5. Summary
The effort and methodology of mapping the subtidal zone of the Wadden Sea were differently pronounced in Denmark, Germany and The Netherlands. Generally, the activities show an increase in quality and quantity over the last six years.
In The Netherlands, annual subtidal sampling campaigns mainly focus on blue mussel and Pacific oyster beds. The program is recently extended to cover Ensis leei as well. Although all other sampled macrobenthic species are also recorded, a structural monitoring is needed in addition to the annual mussel survey in the western Wadden Sea and should be extended to the subtidal zone of the eastern Dutch Wadden Sea. It is therefore recommended to set up a basic monitoring program covering the entire subtidal area over important environmental gradients, which determine species composition, such as sediment type, seabed morphology and current velocity. The additive advantage of such a monitoring program is exemplified by the sampling campaign within the ‘Waddenmozaïek’ project, in which the subtidal Dutch Wadden Sea was sampled in a 1-km grid. This sampling campaign is scheduled to be repeated in 2022 but is not yet included in a long-term monitoring program.
In the Danish Wadden Sea, the focus is also on mapping blue mussel and oyster beds. After almost ten years of very limited mapping activity in the Wadden Sea, a new blue mussel and Pacific oyster stock assessment was carried out in 2017-2018. It turned out that denser stocks of blue mussels are rare and blue mussels always co-occur with oysters at all sampling sites. It is recommended that a regular habitat monitoring program covering the subtidal and tidal areas is initiated within the Danish Wadden Sea.
In the previous reporting period, comprehensive hydroacoustic survey methods (side-scan sonar, multibeam echo sounder) with supplementing sampling were mainly used in Germany for habitat detection. Based on this approach, sediment distribution, seabed morphology as well as habitats and reef structures could be detected and evaluated. Using this approach, dense beds of Pacific oysters living on soft sediment and dense stocks of the invasive algae Sargassum muticum were detected as new habitat types for German waters. In general, sandy areas cover by far the largest part of the subtidal sea floor. Although not evaluated yet, this most probably applies for the entire Wadden Sea. Because of ongoing sediment dynamics, and associated bedforms, this habitat is used by a relatively small number of species. Hard substrates, geogenic and especially biogenic reef structures, occupy significantly less area. However, here the species diversity is significantly higher making them particularly valuable habitats that should be highly protected.
The assessment of habitats and species composition and their variability over space and time is subject of ongoing research. For a better understanding of the functioning of the trilateral subtidal Wadden Sea, much can be gained from combining the best practices on species sampling campaigns and hydroacoustic surveys from the individual countries and applying them in a combined trilateral survey to unify the knowledge on the subtidal Wadden Sea.
About the authorsK. Ricklefs1, O. Franken2,3, S. Glorius4, F. Mascioli5, P. Nielsen6, H.-C. Reimers7, A. Trampe1
1 Research and Technology Centre Westcoast of Kiel University, Hafentörn 1, 25761 Büsum, DE 2 Conservation Ecology Group, Groningen Institute for Evolutionary Life Sciences, University of Groningen, Nijenborgh 7, 9747 AG Groningen, NL 3Department of Coastal Systems, Royal Netherlands Institute for Sea Research, Landsdiep 4, 't Horntje (Texel), 1797 SZ, NL |
References
Baptist, M.J., van der Wal, J.T., Folmer, E.O., Gräwe, U., Elschot, K. (2019) An ecotope map of the trilateral Wadden Sea. J Sea Res 152:101761. doi: 10.1016/j.seares.2019.05.003
Baptist, M.J., van der Wal, J.T., Gräwe, U., Folmer, E., Elschot, K. (2021) Data for: An ecotope map of the trilateral Wadden Sea. Mendeley Data, V2, doi: 10.17632/27mysx289g.2
Capperucci, R.M., Kubicki, A., Holler, P. Bartholomä, A. (2020) Sidescan sonar meets airborne and satellite remote sensing: challenges of a multi-device seafloor classification in extreme shallow water intertidal environments. Geo-Marine Letters, 40(7), 117-133.
Capperucci, R., Bartholomä, A., Bungenstock, F., Enters, D., Karle, M., & Wehrmann, A. (2022) The WASA core catalogue of Late Quaternary depositional sequences in the central Wadden Sea – A manual for the core repository. Netherlands Journal of Geosciences, 101, E5. doi:10.1017/njg.2022.1
Dahl K., Petersen, J.K., (eds) Definition af biogene rev. Miljøstyrelsen. Miljøprojekt nr. 1992 (in Danish).
Davies, C. E., D. Moss & M. O. Hill (2004) EUNIS habitat classification revised 2004. EEA-Report, 310 pp.
Degraer, S., Moerkerke, G., Rabaut, M., van Hoey, G., du Four, I., Vincx, M., Henriet, J.-P., van Lancker, V. (2008) Very-high resolution side scan sonar mapping of biogenic reefs of the tube-worm Lanice conchilega. Remote Sensing of Environment 112(8): 3323-3328.
EC (2013) Interpretation manual of European Union Habitats. European Commission. DG Environment. Nature and biodiversity: 144 pp.
Essink, K. (1985) On the occurrence of the American jack-knife clam Ensis directus (Conrad, 1843) (Bivalvia, Cultellidae) in the Dutch Wadden Sea. Basteria 49: 73-80.
European Environment Agency (2020) Natura 2000 Network Viewer - https://natura2000.eea.europa.eu/
Evans, D., Aish, A., Michez, N., Boon, A.,Condé, S.,Richard, D., Conner, D., Gelabert, E.,Parra, M., Salvati, E., Tunesi, L. (2016) Revising the marine section of the EUNIS Habitat classification - Report of a workshop held at the European Topic Centre on Biological Diversity, 12 & 13 May 2016 - ETC/BD Working paper N°A/2016 revised 2017
Folmer, E., Büttger, H., Herlyn, M., Markert, A., Millat, G., Troost, K., & Wehrmann, A. (2017). Wadden Sea Quality Status Report: Beds of blue mussels and Pacific oysters. Common Wadden Sea Secretariat. https://doi.org/10.5281/zenodo.15193629
Glorius, S.T., Meijboom, A. (2020) Ontwikkeling van de bodemdiergemeenschap in de geulen van referentiegebied Rottum. Tussenrapportage 14 jaar na sluiting (najaar 2019). WMR-Rapport C109/20, pp 50.
Heinrich, C., Feldens, P., Schwarzer, K. (2017) Highly dynamic biological seabed alterations revealed by side scan sonar tracking of Lanice conchilega beds offshore the island of Sylt (German Bight). Geo-Mar Lett 37:289–303.
Holthuijsen, S. (2019) Waddenmozaïek cruise report bemonstering sublitoraal Nederlandse Waddenzee 2019. NIOZ rapportnummer 2019.06.20.01.
ICES (2021) EU request on how management scenarios to reduce mobile bottom fishing disturbance on seafloor habitats affect fisheries landing and value ICES Advice 2021 – sr.2021.08 – https://doi.org/10.17895/ices.advice.8191
Kostylev, V. E., B. J. Todd, G. B. J. Fader, R. C. Courtney, G. D. M. Cameron & R. A. Pickrill (2001) Benthic habitat mapping on the Scotian Shelf based on multibeam bathymetry, surficial geology and sea floor photographs. Marine Ecology Progress Series, 210: 121–137.
Mascioli, F., Bremm, G., Bruckert, P., Tants, R., Dirks, H., & Wurpts A. (2017) The contribution of geomorphometry to the study of tidal inlets. Zeitschrift für Geomorphology, 61/2: 179-197.
Mascioli, F., Piattelli, V., Cerrone, F., Gasprino, D., Kunde, T. & Miccadei E. (2021) Feasibility of Objective Seabed Mapping Techniques in a Coastal Tidal Environment (Wadden Sea, Germany). Geosciences, 11: 49, https://doi.org/10.3390/geosciences11020049.
Miljøstyrelsen (2020) Natura 2000-basisanalyse 2022 – 2027 Vadehavet.
OSPAR Commission (2017) Extent of Physical Damage to Predominant and Special Habitats. https://oap.ospar.org/en/ospar-assessments/intermediate-assessment-2017…
Nielsen, P., Geitner, K., Jakobsen, J., Köppl, C. J., & Petersen, J. K. (2019) Fagligt grundlag for forvaltningsplan for udvikling af bæredygtige fiskerier af muslinger og østers i Vadehavet. Institut for Akvatiske Ressourcer, Danmarks Tekniske Universitet. DTU Aqua-rapport Nr. 334-2018 (in Danish).
Ricklefs, K., Büttger, H., Asmus, H. (2020) Occurrence, stability, and associated species of subtidal mussel beds in the North Frisian Wadden Sea (German North Sea Coast). Estuarine, Coastal and Shelf Science 233, 106549
Ricklefs, K., Trampe, A. (2021) Beschreibung der Vorgehensweise bei der sonarbasierten Detektion und Kartierung sublitoraler Muschelhabitate, Sedimente und "Riff-Strukturen" im Schleswig-Holsteinischen Wattenmeer. Tech. Report FTZ Westküste, DOI: 10.13140/RG.2.2.18615.85921.
Schaumann, R., Capperucci, R.M., Bungenstock, F., McCann, T., Enters, D., Wehrmann, A., Bartholomä, A. (2022) The Middle Pleistocene to early Holocene subsurface geology of the Norderney tidal basin: new insights from core data and high-resolution sub-bottom profiling (Central Wadden Sea, southern North Sea). Netherlands Journal of Geosciences, 100, E15. doi:10.1017/njg.2021.3.
Schückel, U., Martinez Arbizu, P., Kuru, S., Machnis, A., Schückel, s. (submitted): Leben und Vielfalt im Sublitoral des Wattenmeeres – das Hörnum Tief als Hotspot der Biodiversität, Jagd und Artenschutz, Jahresbericht 2021: Zur biologischen Vielfalt.
Schwemmer, P., Adler, S., Enners, L., Volmer, H., Kottsieper, J., Ricklefs, K., Stage, M., Schwarzer, K., Wittbrodt, K., Reimers, H.-C., Binder, K., Asmus, R., Asmus, H., Horn, S., Schückel, S., Kohlus, J., Eskildsen, K., Klingbeil, K., Gräwe, U., Garthe S, (2019) Modelling and predicting habitats for the neobiotic American razor clam Ensis leei in the Wadden Sea: a matter of scale. Estuarine, Coastal and Shelf Science 231, 106440
Troost, K., M. van Asch, E. Brummelhuis, D. van den Ende, Y. van Es, K.J. Perdon, J. van der Pool, C. van Zweeden & J. van Zwol (2021) Schelpdierbestanden in de Nederlandse kustzone, Waddenzee en zoute deltawateren in 2020. CVO report 21.001, pp 96.
Troost, K., J. van der Meer, M. van Stralen (2022) The longevity of subtidal mussel beds in the Dutch Wadden Sea. Journal of Sea Research 181.
Tulp, I., Craeymeersch, J., Leopold, M., van Damme, I., Fey, F., Verddat, H. (2010) The role of the invasive bivalve Ensis directusas food source for fish and birds in the Dutch coastal zone. Estuarine, Coastal and Shelf Science 90: 116-128.
Valentine, P. C., B. J. Todd & V. E. Kostylev (2005) Classification of marine sublittoral habitats, with application to the northeastern North America region. American Fisheries Society Symposium 41: 183-200.
Vorberg, R. (1995) On the decrease of sabellarian reefs along the German North Sea coast. - Publ. Serv. Géol. Lux. 29: 87-93.
Vorberg, R. (2005) Sabellaria Reefs. In: Essink, K., Dettman, C., Farke, H., Laursen, K., Lüerßen, G., Marencic, H. & Wiersinga, W.: Wadden Sea Quality Status Report 2004. Wadden Sea Ecosystem No. 19. Trilateral Monitoring and Assessment Group, Common Wadden Sea Secretariat, Wilhelmshaven, Germany. 208-210.
Vorberg, R., F. Fey, & J. Jansen (2009) Subtidal habitats. In: Marencic, H. & J. de Vlas (eds.). 2009. Quality Status Report 2009. WaddenSea Ecosystem No. 25. Common Wadden Sea Secretariat, Trilateral Monitoring and Assessment Group, Wilhelmshaven, Germany.
Vorberg, R., Glorius, S., Mascioli, F., Nielsen, P., Reimers, H.-C., Ricklefs, K., & Troost, K. (2017). Wadden Sea Quality Status Report: Subtidal habitats. Common Wadden Sea Secretariat. https://doi.org/10.5281/zenodo.15209673
This report should be cited as: Ricklefs, K., Franken, O., Glorius, S., Mascioli, F., Nielsen, P., Reimers, H.-C., & Trampe, A. (2022). Wadden Sea Quality Status Report: Subtidal habitats. Common Wadden Sea Secretariat. https://doi.org/10.5281/zenodo.15209437
All 2022 reports may be cited collectively as: Kloepper, S., Bostelmann, A., Bregnballe, T., Busch, J.A., Buschbaum, C., Deen, K., Domnick, A., Gutow, L., Jensen, K., Jepsen, N., Luna, S., Meise, K., Teilmann, J., & van Wezel, A. (2022). Wadden Sea Quality Status Report. Common Wadden Sea Secretariat, Wilhelmshaven, Germany. Downloaded DD.MM.YYYY. qsr.waddensea-worldheritage.org
