Photo: Jan Drent. Common cockle (Cerastoderma edule) inhalant and exhalent siphons.
Macrozoobenthos
J. Drent, R. Bijkerk, M. Herlyn, M. Grotjahn, J. Voß, M.-C. Carausu, D. W. Thieltges
Published 2017
1. Introduction
Macrozoobenthos is practically defined as the invertebrate community living in or on the sediment or hard substrates and retained on a 1 mm2 mesh sieve. Macrozoobenthos species in the Wadden Sea food web can be categorized as herbivores, detritivores and carnivores (Asmus & Asmus, 1985). Tracing carbon flows has shown that the macrozoobenthos compartment largely depends on phytoplankton (harvested by suspension feeders) and benthic microalgae (microphytobenthos, fed upon by deposit feeders) and is therefore mainly herbivorous (Van Oevelen et al., 2006). Because only few other metazoan species harvest benthic and pelagic primary production, macrozoobenthic species are the most important secondary producers of the Wadden Sea ecosystem constituting an important food source for large numbers of birds and fish in the area (Dankers et al., 1983; Reise et al., 2010).
Besides this key role as link to higher trophic levels in the food web macrozoobenthos also contributes to other ecosystem functions (Olff et al., 2009). Deposit feeding and bioturbation by macrozoobenthos, for example, changes the sediment composition, whereas irrigation enhances the exchange of oxygen and other elements between the water and the sediment. Suspension feeders transfer suspended matter from the water column to the sediment (Verwey, 1952) and dense beds of epibenthic bivalves form biogenic structures that provide habitat for numerous associated species (Gutiérrez et al., 2003).
Worldwide anthropogenic influences, such as eutrophication, non-nutrient pollutants, species invasions, overfishing, habitat alteration and climate change, are impacting ecosystem processes in coastal seas (e.g., Levin et al., 2001). These factors have also been discussed for the Wadden Sea in previous Wadden Sea Quality Status Reports (1999, 2004 and 2009) but remain relevant today.
An increase of macrozoobenthos biomass in the western Wadden Sea was linked to eutrophication, due to increasing nutrient loads until the 1980s (Beukema et al., 2002). Currently, nutrient loads are decreasing and may cause secondary production and biomass of macrozoobenthos to decline (Philippart et al., 2007). A warming climate and in particular milder winters are expected to improve winter survival of many species, whereas bivalve recruitment is expected to suffer from a higher abundance of epibenthic predators after warm winters (Beukema 1992; Beukema & Dekker 2005; Strasser et al., 2003). Mainly in the last few decades several invasive species have established in the Wadden Sea which may impact native species (Buschbaum et al., 2012, report on "Alien species"). Finally, these large-scale drivers as well as local effects like bottom trawling and shellfisheries could potentially change species composition with far reaching effects on ecosystem functioning (Eriksson, 2010).
In this report, we will use available long term monitoring series in the Wadden Sea (Figures 1 and 2) from 1989 onwards to 1) investigate the long-term development of the macrozoobenthos standing stock that might have responded to changes in the eutrophication levels and other drivers; 2) examine the macrozoobenthos species composition and trends in functional composition; 3) assess the impact of invasive species as the proportion of the standing stock and 4) show trends in numerical densities of most important species.
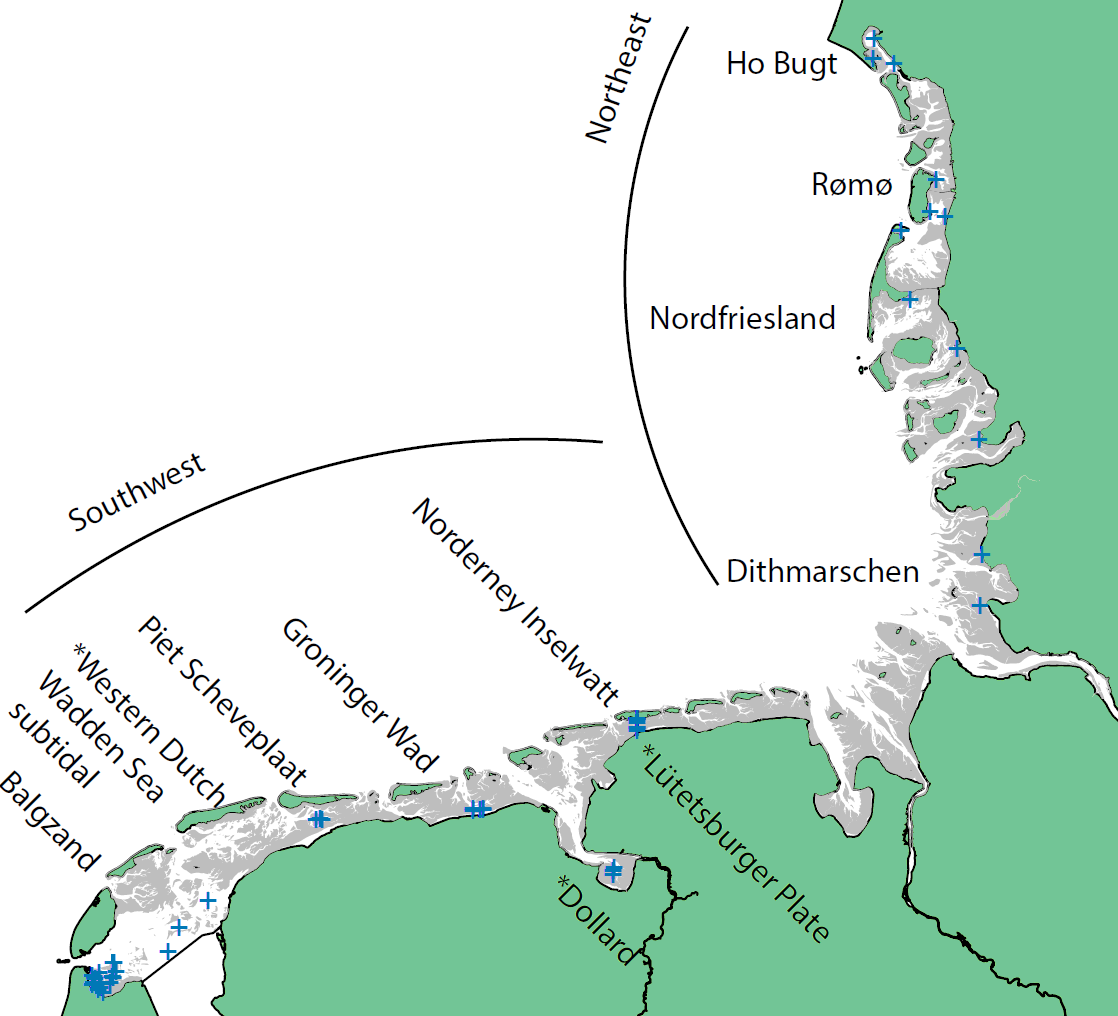 Figure 1. Macrozoobenthos monitoring areas in the Wadden Sea divided into two sub regions Northeast and Southwest. Monitoring areas marked with * are not included in Wadden Sea and subregion averages. Positions of sampling stations or transects within monitoring areas are indicated with blue + marks. All transects and stations are located in the intertidal except the monitoring area Western Dutch Wadden Sea which consists of three subtidal transects. More details about the monitoring areas and sampling schemes are included in Annex 1.
Figure 1. Macrozoobenthos monitoring areas in the Wadden Sea divided into two sub regions Northeast and Southwest. Monitoring areas marked with * are not included in Wadden Sea and subregion averages. Positions of sampling stations or transects within monitoring areas are indicated with blue + marks. All transects and stations are located in the intertidal except the monitoring area Western Dutch Wadden Sea which consists of three subtidal transects. More details about the monitoring areas and sampling schemes are included in Annex 1.
 Figure 2. Left: Macrozoobenthos sampling in action at Balgzand. Monitoring started here long before hand held gps devices were available and the locations of the 50 stations along the 12 transects were found with a measuring wheel. This method is still used today. Stations are spaced 20 meters apart along the 12 transects of the monitoring series. (Photo: Lodewijk van Walraven). Right: Crucibles aligned for determination of ash free dry mass of the macrozoobenthos (photo: Jan Drent).
Figure 2. Left: Macrozoobenthos sampling in action at Balgzand. Monitoring started here long before hand held gps devices were available and the locations of the 50 stations along the 12 transects were found with a measuring wheel. This method is still used today. Stations are spaced 20 meters apart along the 12 transects of the monitoring series. (Photo: Lodewijk van Walraven). Right: Crucibles aligned for determination of ash free dry mass of the macrozoobenthos (photo: Jan Drent).
2. Status and trends
2.1 Biomass
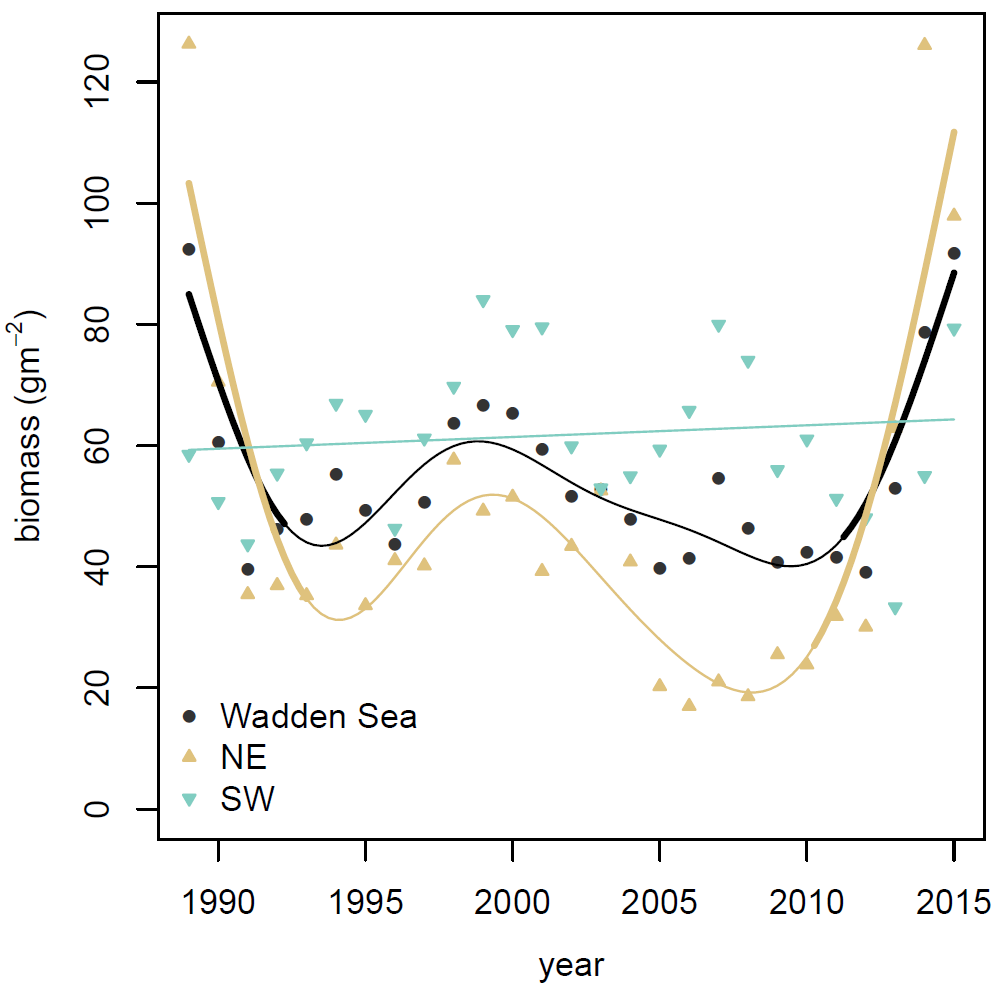 Figure 3. Total average biomass (ash-free dry mass) of the macrozoobenthos in the entire Wadden Sea and in the two sub regions Northeast (NE) and Southwest (SW). Significant temporal trends are indicated by bold line type.
Figure 3. Total average biomass (ash-free dry mass) of the macrozoobenthos in the entire Wadden Sea and in the two sub regions Northeast (NE) and Southwest (SW). Significant temporal trends are indicated by bold line type.
On a Wadden Sea wide scale there have been no significant long term temporal trends in the standing stock of the macrozoobenthos since 1989 and therefore also no changes of the status since the previous Quality Status Reports.
The total macrozoobenthos standing stock averaged over eight intertidal monitoring areas in the Wadden Sea that were sampled at least since 1989 and remained between 40 and 60 gm-2 during most years (Figure 3). This deviated at the beginning and the end of the series where biomass values were significantly increased. At the level of the two subregions only in the Northeastern (NE) Wadden Sea these upward deviations took place, due to large biomass contributions of the cockle Cerastoderma edule (Figure 4) and the sand gaper Mya arenaria, while in the Southwestern (SW) Wadden Sea biomass was stable during the entire observed period.
The average biomass in the NE Wadden Sea was about 75 % of SE Wadden Sea average. This difference in the long-term average biomass may be related to regional patterns in chlorophyll concentration and eutrophication status. In the NE Wadden Sea the summer chlorophyll concentrations and eutrophication status are lower than in the SW Wadden Sea (Van Beusekom et al., 2009), suggesting lower food availability for the macrozoobenthos. While this may explain the lower macrozoobenthos standing stock in the NE Wadden Sea, the differences in the temporal patterns between the two regions do not reflect the general trend of slowly decreasing chlorophyll concentrations and eutrophication status of the Wadden Sea (Van Beusekom et al., 2009). This is interesting as eutrophication has been mentioned as an important driver of long-term changes of the macrozoobenthos species composition in the Wadden Sea (Schückel & Kröncke, 2013; Schumacher et al., 2014) and in particular for changes in macrozoobenthos biomass (Beukema et al., 2002), also during the recent de-eutrophication (Philippart et al., 2007). However, this perception is mainly based on local trends at Balgzand in the SW Wadden Sea and can apparently not be extrapolated to other areas (De Jonge & Essink. 1991; see Figures Annex 2). Interestingly, although nutrient input is declining the long term positive trends of macrozoobenthos biomass measured in spring at Balgzand and in the nearby subtidal of the western Dutch Wadden Sea are still continuing (see Figures Annex 2). These observations question the general relevance of the eutrophication status for the biomass dynamics and point to the fact that the macrozoobenthos standing stock is the result of complex food web interactions (e.g., Polis & Strong, 1996) and recruitment variability (Van der Meer et al., 2001). Complexity of the food web is considerable, because not only primary production but also consumers of macrozoobenthos impact standing stock of the benthic invertebrates. Besides that excess of primary produced organic matter due to eutrophication is not necessarily consumed by the macrozoobenthos but is likely also processed by the microbial food web (Van Oevelen, 2006). In addition to biological interactions abiotic stressors like low winter temperatures can cause mortality and lead to between annual variability (Möller, 1986).
 Figure 4. Left: Cerastoderma edule inhalant and exhalent siphons; right: Capitallidae member Heteromastus filiformis (photos: Jan Drent).
Figure 4. Left: Cerastoderma edule inhalant and exhalent siphons; right: Capitallidae member Heteromastus filiformis (photos: Jan Drent).
2.2 Species composition and ecological function
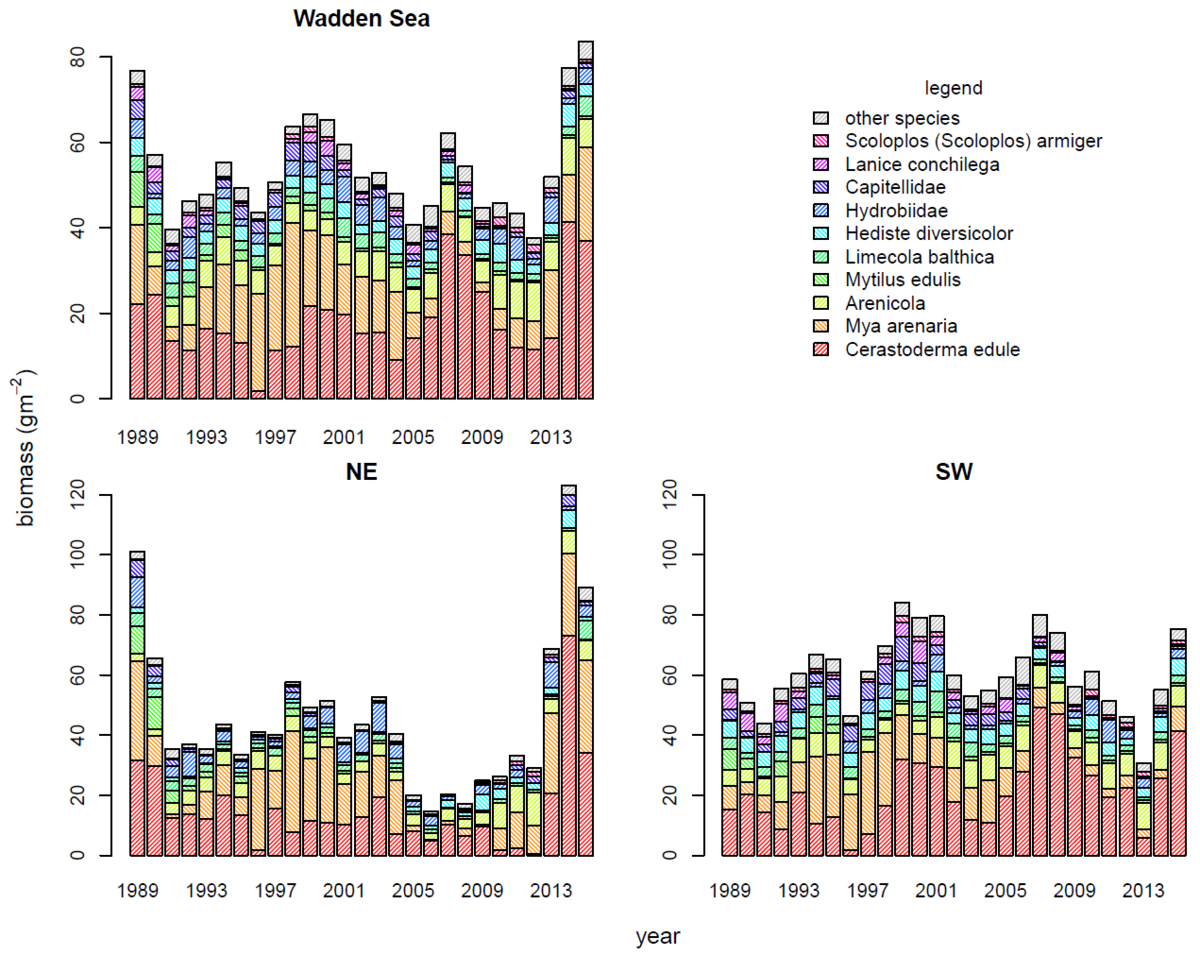 Figure 5: Contribution of the ten most common species or taxonomic groups to the total biomass (ash-free dry mass) in the entire Wadden Sea and in the Northeastern (NE) and southwestern (SE) sub regions. A further specification per monitoring area can be found in annex 3.
Figure 5: Contribution of the ten most common species or taxonomic groups to the total biomass (ash-free dry mass) in the entire Wadden Sea and in the Northeastern (NE) and southwestern (SE) sub regions. A further specification per monitoring area can be found in annex 3.
Some changes in species composition were observed but these did not translate to higher level taxonomic or functional shifts in the community as a whole.
Macrozoobenthos biomass was unevenly distributed among species. Only ten species (less than 10 % of the species pool) are responsible for more than 90 % of the total biomass (Figure 5). The cockle, Cerastoderma edule, was the major contributor to total biomass, with a 35 % share of the Wadden Sea wide long-term average (1989-2015). The sand gaper, Mya arenaria, and the lugworm, Arenicola spp., followed with long-term biomass contributions of 22 % and 11 %, respectively.
The Northeastern (NE) Wadden Sea and Southwestern (SW) Wadden Sea have rather similar species composition with a few notable differences. The cockle dominance was comparable in both subregions, while the sand gaper contribution in the NE Wadden Sea (30 %) was much larger than in the SW Wadden Sea (17 %). This was mainly due to a very strong presence of M. arenaria in the Dithmarschen monitoring area (Figure Annex 3), probably influenced by fresh water inflow from Elbe and Eider. Like M. arenaria also mud snails (Hydrobiidae) are stronger represented in the NE Wadden Sea (8 %) than in the SW Wadden Sea (4 % of the total biomass). In the SW Wadden Sea the ragworm Hediste diversicolor (8 % versus 4 %) and the Capitellidae (5 % versus 2 %) were relatively more important.
Temporally the dominance of cockles has slightly increased mainly in the SW Wadden Sea, but was interrupted in 1996 in the entire Wadden Sea coinciding with the coldest winter in the time span considered here. The relative winters of 2010 and 2011 matched with a period of low cockle biomass in the NE Wadden Sea. In the SW Wadden Sea there was low cockle dominance in 2013 after an exceptionally cold spring.
Before 2000, the most important change in species composition was the declining contribution of the blue mussel Mytilus edulis. This change happened both in the NE and the SW Wadden Sea and up to 2015 no recovery has been observed (see "Beds of blue mussels and Pacific oysters"). Similarly, the proportional biomass of the sand mason worm Lanice conchilega showed a long term declining trend in the Wadden Sea species pool.
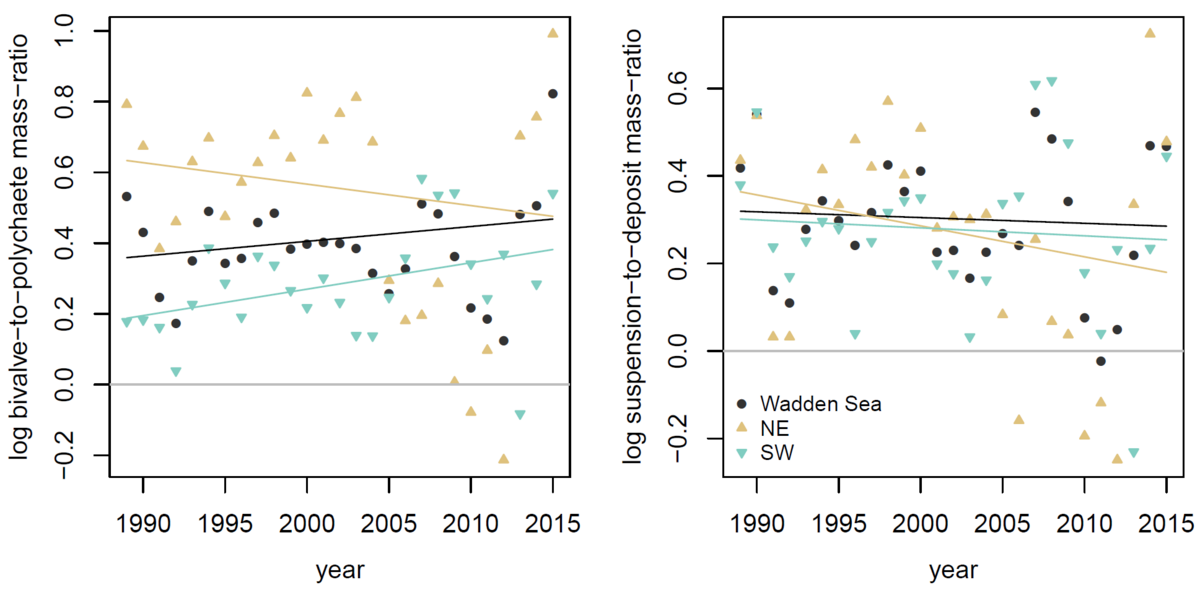 Figure 6. Bivalve-to-polychaete mass-ratio (left) and suspension-to-deposit-feeder mass ratio (right) in the Wadden Sea and the Northeastern (NE) and Southwestern (SW) subregions. None of the trends were significant. The grey line is the line of equal contributions.
Figure 6. Bivalve-to-polychaete mass-ratio (left) and suspension-to-deposit-feeder mass ratio (right) in the Wadden Sea and the Northeastern (NE) and Southwestern (SW) subregions. None of the trends were significant. The grey line is the line of equal contributions.
The observed changes in species composition did not translate into long-term shifts in taxonomic or functional composition quantified as the bivalve-to-polychaete mass-ratio, or the suspension-to-deposit-feeder mass ratio (Figure 6). Based on data collected over more than one century in the NE Wadden Sea Reise (1982) suggested that polychaetes were gaining in abundance compared to other taxonomic groups. This observation was discussed together with new data in previous Quality Status Reports and led to the hypothesis that changing species composition and consequently declining functional dominance of stabilizing bivalve filter feeders and increasing destabilizing polychaete deposit feeders have caused large impacts on the Wadden Sea ecosystem (Eriksson et al., 2010). However, recent data from various monitoring areas in the Wadden Sea do not show an ongoing increase in dominance of polychaetes or large shifts in functional groups.
2.3 Invasive species
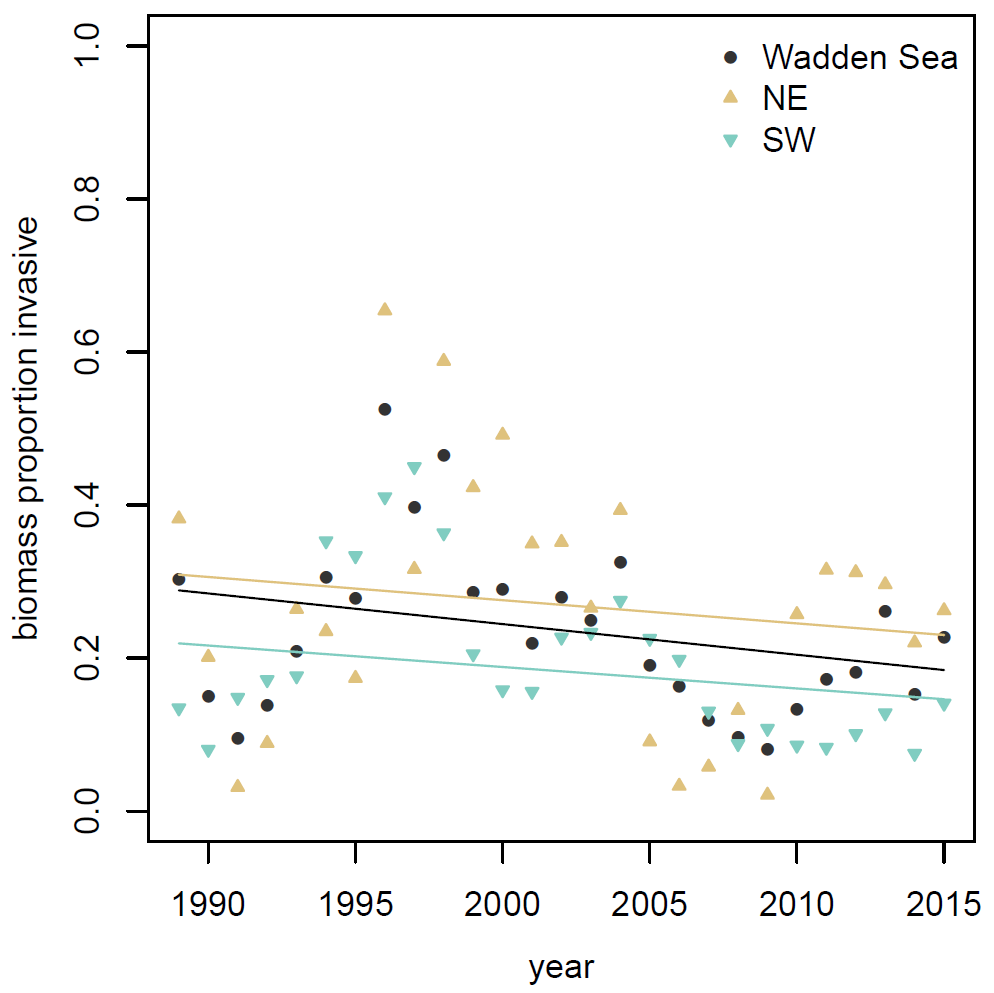 Figure 7. Proportional contribution of invasive species to the total macrozoobenthos biomass in the Wadden Sea and in the Northeastern (NE) and Southwestern (SE) sub regions. None of the trends were significant.
Figure 7. Proportional contribution of invasive species to the total macrozoobenthos biomass in the Wadden Sea and in the Northeastern (NE) and Southwestern (SE) sub regions. None of the trends were significant.
Since the previous QSR there has been little change in presence and dominance of invasive species in the macrozoobenthos monitoring series in the Wadden Sea.
The biomass contribution by invasive species in the Wadden Sea did not show significant trends in the time span since 1989 (Figure 7). However, between 1995 and 2000 data showed a period of rather high dominance of invasive biomass (Figure 7). Both in the Northeastern (NE) and Southwestern (SE) Wadden Sea this higher invasive proportion was mainly attributable to the development of the sand gaper Mya arenaria, which is an established ancient invader from 400 to 700 years ago (Strasser, 1999) and no threat to the present ecosystem. During the last ten years invasive species made up an average of 16 % of total biomass in the Wadden Sea without significant trends. In this period the proportion of invasive biomass was slightly higher in NE Wadden Sea than in the SW Wadden Sea.
Most prominent recent invasive species are the Pacific oyster, Crassostrea gigas, the Atlantic razor clam, Ensis leei and the spionid worm Marenzelleria viridis. All these species are established for more than a decade now and have been discussed in the previous Quality Status Report. Except for E. leei none of these species ranked among the twenty most important macrozoobenthos species from the intertidal flats based on occurrence and biomass compiled for the present QSR (Box 4). However, these invasive species have become very dominant at specific sites (Annex 4) such as in the estuarine Dollard, the subtidal of the Western Dutch Wadden Sea and on blue mussel beds. The Pacific oyster, Crassostrea gigas, has settled in existing mussel beds (Reise, 1998), for example at the monitoring area on the Lütetsburger Plate. An important feature of C. gigas is that it provides a reef-like hard substrate habitat for a range of species including the invasive crab Hemigrapsus takanoi and H. sanguineus. Many former mussel beds are now mixed beds of oysters and mussels (Nehls et al., 2006, see report on "Beds of blue mussels and Pacific oysters"). The razor clam Ensis leei attained very high biomass values on the fringe between intertidal and subtidal at Balgzand and also in the subtidal (Dekker & Drent, 2013; Dekker & Beukema, 2012). Previously macrozoobenthos biomass in these habitats was low and it seems that Ensis leei is filling an earlier unused niche in the system (Dekker & Beukema, 2012).
In the Wadden Sea the spionid Marenzelleria viridis was first found in the Dollard in 1983, where it went through a boom and burst phase around 1990 and locally doubled the total macrozoobenthos biomass (Essink & Dekker, 2002). During that time it also appeared at other sites in the Wadden Sea. It developed a large biomass in the brackish subtidal of the western Dutch Wadden Sea (Dekker & Drent, 2013). However, M. viridis appears to have remarkably little impact on the local community, except for reports mentioning a negative impact on the ragworm Hediste diversicolor (Delefosse et al., 2012) (see report on "Alien species").
2.4 Numerical densities/population developments
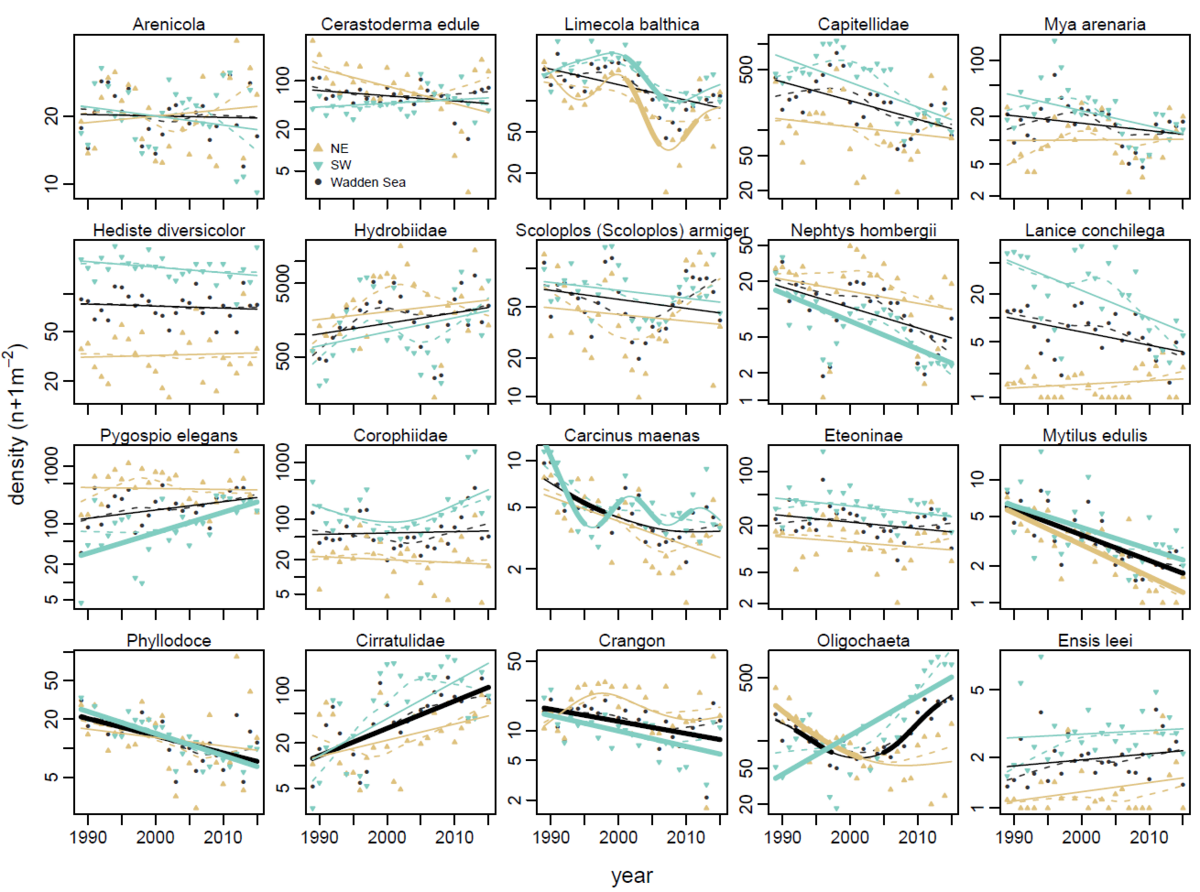 Figure 8. Temporal development of average densities of the twenty most important macrozoobenthos species in the Wadden Sea and the Northeastern (NE) and Southwestern (SW) subregions. Solid lines are GAM fits, dashed lines are LOWESS running means. Significant temporal trends are indicated by bold line type.
Figure 8. Temporal development of average densities of the twenty most important macrozoobenthos species in the Wadden Sea and the Northeastern (NE) and Southwestern (SW) subregions. Solid lines are GAM fits, dashed lines are LOWESS running means. Significant temporal trends are indicated by bold line type.
Macrozoobenthos species varied in density over time and there were marked differences among the monitoring areas.
Numerical densities of macrozoobenthos species varied strongly through time (Figure 8). Between 1989 and 2015 nine of the twenty most dominant species (in occurrence and biomass) showed significant long-term trends and/or short-term fluctuations, either Wadden Sea wide or within the NE or SW regions. Declining species highlighted in the previous Quality Status Report (Van der Graaf et al., 2009), such as Limecola balthica, Scoloplos armiger and Heteromastus filiformis (now included in the Capitellidae) (Figure 4), all showed recovery since their lows five to ten years ago. The recovery of L. balthica in the western Dutch Wadden Sea has been linked to the ban on mechanical cockle fisheries (Compton et al., 2016). However, the recent upward trend of L. balthica abundance seems to be Wadden Sea wide and is not restricted to the area intensively dredged by mechanical cockle fisheries.
Consistent declining trends including both the NE and SW Wadden Sea were observed for Mytilus edulis. These trends are probably indicative of a long-term poor recruitment of this species, further covered in the report "Beds of blue mussels and Pacific oysters".
Pygospio elegans, the family Cirratulidae with invasive genus members Tharyx and Aphelochaeta, and oligochaetes increased in the SW Wadden Sea. Oligochaetes are associated with organically rich sediment, are tolerant to a wide range of environmental conditions (Giere & Pfannkuche, 1982) and may indicate disturbed habitat (Borja et al., 2000).
Nephtys hombergii (a carnivorous polychaete) and Lanice conchilega (a tube worm that can form reefs; De Smet et al., 2013) are both sensitive to low winter temperatures. Densities of these species clearly dropped after the cold winters of 1996 and 2010-2012. In contrast to expectations they did not respond with increasing trends to high frequencies of mild winters in the past two decades (Van der Meer et al., 2013).
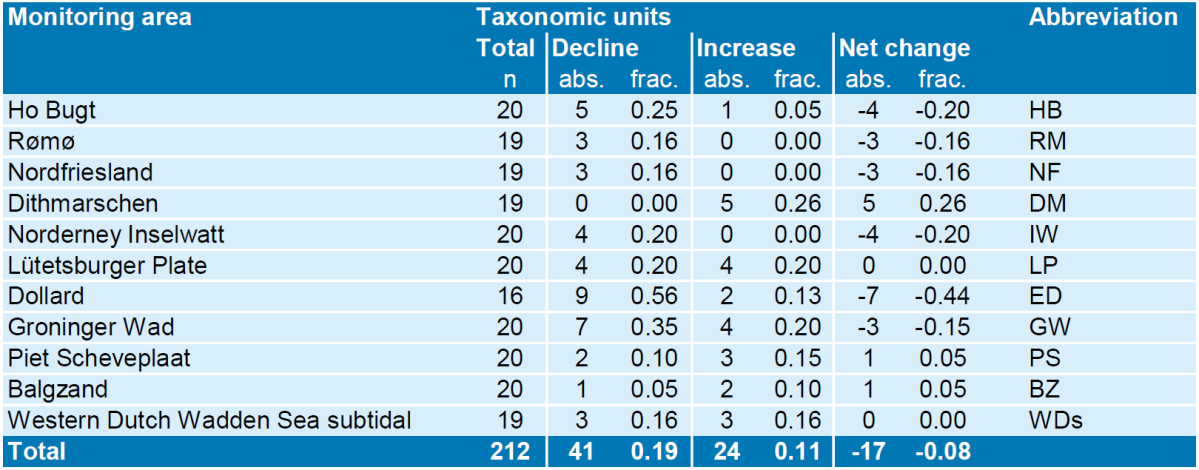
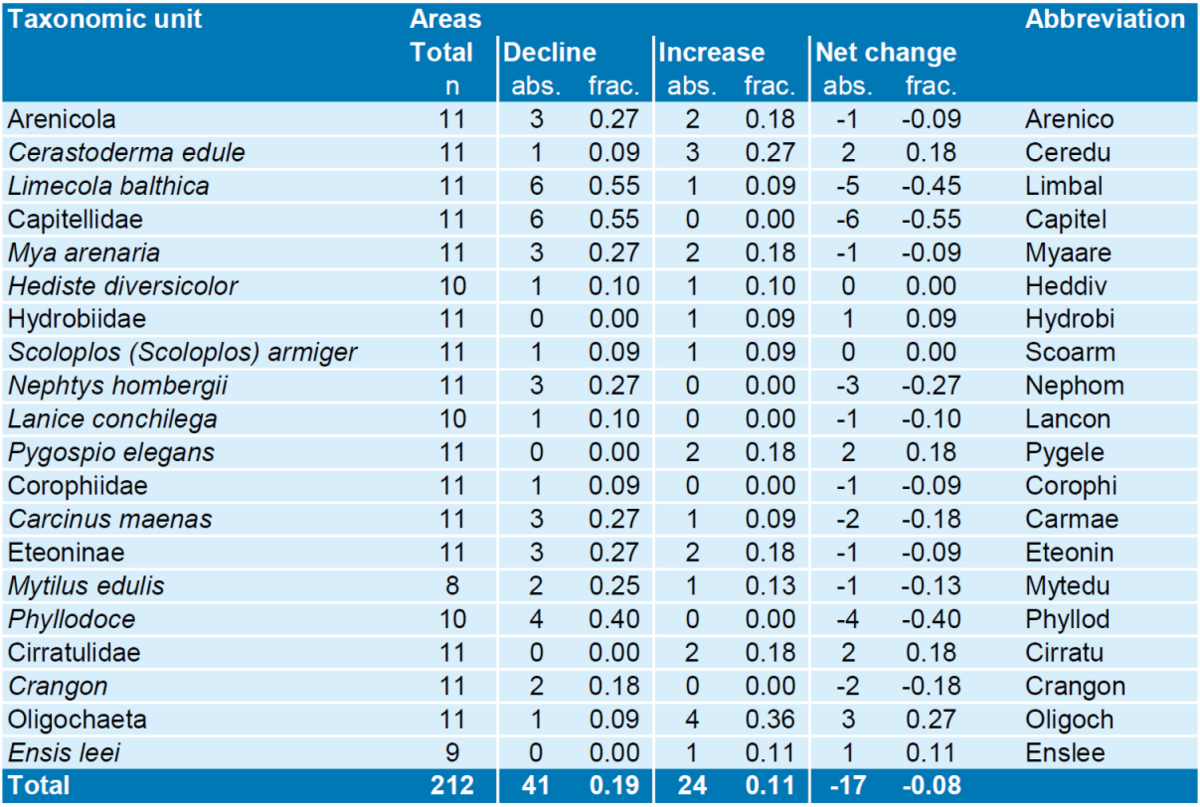
At the level of local monitoring areas, patterns of species density developments often diverged from the average patterns (Annex 5). A summary of significant density trends between 2005 and 2015 per species and location (Table 1 & 2, for figures see annex 5) indicates that of the 212 available species-area combinations there were 41 with negative and 24 with positive trends during the last ten years. Most negative trends were found in the Dollard, where nine out of 16 locally occurring dominant Wadden Sea macrozoobenthos species decreased, while only two increased (see Annex 6 for more details).
The taxon that declined in the largest number of areas was the Capitellidae, with six declines and no increases in the remaining five areas (Table 2). Also Limecola balthica became less abundant in six areas (although with a recent recovery not yet breaking the trend) and increased only in one. Phyllodoce spp. decreased in four areas and increased in none. Another species with many drops and no increases was Nephtys hombergii, declining in three areas and increasing in none. The taxon with most positive trends was the Oligochaeta, increasing at four areas and decreasing at one.
Table 1. Summary of significant temporal trends in density of the 20 most important taxonomic units between 2005 and 2015 at 11 monitoring areas throughout the Wadden Sea. Note that not all species occur in every monitoring area leading to lower total n. Given are the absolute (abs.) number of observed significant trends of the taxonomic units (separate for declines and increases) as well as their fraction (frac.). Net change is increase minus decline.
Table 2. Summary of significant trends in density between 2005 and 2015 at 11 monitoring areas in the Wadden Sea for each of the 20 most important macrozoobenthos taxonomic units. Note that not all taxonomic units occur in each monitoring area and therefore reducing the total number of areas for these taxonomic units. Given are the absolute (abs.) number of areas where a taxonomic unit showed a significant trend (separate fro declines and increases) as well as their fraction (frac.) in relation to the total number of monitoring areas where the taxonomic unit was observed. Net change is increase minus decline.
3. Assessment
No specific trilateral target was developed for the macrozoobenthos of soft sediments in the tidal area of the Wadden Sea. However, the general target of an increased area of geomorphologically and biologically undisturbed tidal flats and subtidal areas applies (Van der Graaf et al., 2009). Wadden Sea wide the macrozoobenthos is relatively stable in numbers, biomass and species composition. However, the blue mussel Mytilus edulis was declining, although this species is not optimally covered in the present design of macrozoobenthos monitoring. There were no consistent effects of eutrophication and more recent de-eutrophication or oligotrophication on the overall Wadden Sea intertidal macrozoobenthos community. Nevertheless, in the Water Framework Directive assessment of German coastal and transitional waters, where the status of the macrozoobenthos is only part of the total assessment, several waterbodies in the Wadden Sea received a moderate or poor ecological status, mainly due to eutrophication (Voß et al., 2010).
Locally there are deviations from the general patterns and especially in the Dollard area we observed many species with decreasing densities. In agreement with this, the Dollard macrozoobenthos received a moderate ecological status in the Water Framework Directive assessment (Van Loon et al., 2015). This may be related to deteriorating environmental conditions like increasing suspended matter concentrations in the water (Van Maren et al., 2016). In addition, locally invasive species have become the dominant contributors to biomass (e.g., in the subtidal and on mussel beds) but they do not contribute more than 10 % on average in other areas of the Wadden Sea.
The present status and the observed trends obviously refer to conditions within the time span of the available time series. Information about the status of the macrozoobenthos before 1970 is available for the western Dutch Wadden Sea (Kraan et al., 2011), the Jade Bay (Schückel & Kröncke, 2013) the Konigshaven (Schumacher et al., 2014) and the Ho Bugt (Jensen, 1992). In addition, a more general picture of long-term human impacts leading to deterioration of the historical Wadden Sea food, of which the macrozoobenthos is an important component, was sketched by Lotze (2005). All these studies show to a smaller or greater extent that the macrozoobenthos community has changed due to climate change, sea level rise, eutrophication and human disturbances like fisheries on benthic populations. Depending on the subjective criteria chosen the Wadden Sea macrozoobenthos could either be considered to be in a poor state (Eriksson et al., 2010) or, in spite of ongoing changes, still seen as an essential part of an ecologically healthy functioning system (Reise et al., 2010). The available time series data since 1970 suggest that changes in the last four decades have been modest and that the macrozoobenthos has remained relatively stable compared to changes suggested to have taken place in the past.
4. Recommendations
A trilateral harmonized macrozoobenthos monitoring effort of at least one measuring campaign each year at every monitoring site should be maintained. Skipping years as was done in recent years at Piet Scheveplaat, Groninger Wad, Rømø and Ho Bugt strongly compromises the quality and value of these long-term time series. Macrozoobenthos dynamics depend strongly on recruitment success that is very variable between years and species. Without continuous yearly measurements the study of macrozoobenthos dynamics is hardly possible and recognizing temporal trends will only be feasible on much longer time scales.
There should be an initiative to develop suitable metrics that relate more closely to ecosystem processes like secondary production by the macrozoobenthos. This would help to improve the assessment of the functioning of the Wadden Sea food web where algal primary production fuels the macrozoobenthos secondary production, which is a basic requirement for the higher trophic levels like the many birds and fish in the ecosystem.
The potential for further harmonization of the trilateral macrozoobenthos monitoring should be explored and measures for intensification of harmonized sampling schemes and data analyses taken.
To improve representativeness of the results of the macrozoobenthos monitoring in the very dynamic and variable environment of the Wadden Sea, spatial coverage should be expanded. This could partly be achieved by including other ongoing national monitoring efforts in the trilateral monitoring programme. This applies in particular to the SIBES monitoring program (Compton et al., 2013) in the Dutch part of the Wadden Sea.
The generally negative macrozoobenthos developments in the Dollard need to be investigated in more detail and research should identify how these developments relate to local environmental drivers or disturbances.
The prevention of new introductions of non-native species is of continuing importance (see report alien species). As impacts are not well studied, research activities should address the effects of non-native species on the macrozoobenthos and general ecosystem processes.
5. Summary
The macrozoobenthos (invertebrates >1 mm living in the sediment or attached to the seafloor) is a crucial component of the Wadden Sea food web. Many of the macrozoobenthos species are herbivores and feed from the phytoplankton in the overlying water or the macrophytobenthos growing on the sediment surface. In turn the macrozoobenthos is the main food source for many bird and fish species of the Wadden Sea. Threats or drivers influencing the macrozoobenthos community are climate change, eutrophication and more recent oligotrophication, invasive macrozoobenthos species, sediment disturbing fisheries and water quality.
Over the last decades total biomass of the macrozoobenthos has been relatively stable with some exceptions in specific monitoring areas where populations of invasive species strongly decreased or increased (in particular the ancient invader Mya arenaria and recent invaders Ensis leeis and Marenzelleria viridis). However, over the entire Wadden Sea the contribution of invasive species to the total biomass has been stable at about 16 % over the last ten years. Similarly, the taxonomic and functional community composition (bivalve-polychaete mass-ratio and suspension-to-deposit-feeder mass-ratio) has been relatively stable over the last decades with no directional trends. At a lower taxonomic level, the average densities of the mussel Mytilus edulis are declining in the Wadden Sea. Limecola balthica and Capitellidae densities show long term declines in several monitoring areas, although in the Southwestern Wadden Sea declines in L. balthica have recently come to an end and populations are increasing again. In many of the monitoring areas in the southern Wadden Sea Nephtys hombergii abundance is declining. When investigating species developments at the different monitoring areas, the Dollard clearly stood out with more than half of the common species declining in abundance, possibly caused by human disturbance.
About the authorsJan Drent1, Ronald Bijkerk2, Marc Herlyn3, Michael Grotjahn3, Joachim Voß4, Mihail-Constantin Carausu5 and David W. Thieltges1 1 NIOZ Royal Netherlands Institute for Sea Research, Department of Coastal Systems, and Utrecht University, P.O. Box 59, 1790 AB, Den Burg, Texel, NL 2 Koeman en Bijkerk Ecologisch Onderzoek en Advies, Postbus 111, 9750 AC Haren, NL 3 NLWKN - Lower Saxony Water Management, Coastal Defence and Nature Conservation Agency, DE 4 Landesamt für Landwirtschaft, Umwelt und ländliche Räume Schleswig-Holstein. Hamburger Chaussee 25, 24220 Flintbek, DE 5 Danish Centre for Environment and Energy (DCE), Department of Bioscience Section for Marine Diversity and Experimental Ecology, Frederiksborgvej 399, PO Box 358, DK-4000 Roskilde, DK |
References
Asmus H. & Asmus R. (1985) The importance of grazing food chain for energy flow and production in three intertidal sand bottom communities of the northern Wadden Sea. Helgoländer Meeresuntersuchungen 39: 273-301.
Beukema J.J. (1992) Expected changes in the Wadden Sea benthos in a warmer world: Lessons from periods with mild winters. Netherlands Journal of Sea Research 30: 73-79.
Beukema J.J., Cadée G.C. & Dekker R. (2002) Zoobenthic biomass limited by phytoplankton abundance: evidence from parallel changes in two long-term data series in the Wadden Sea. Journal of Sea Research 48: 111 –125.
Beukema J.J. & Dekker R. (2005) Decline of recruitment success in cockles and other bivalves in the Wadden Sea: possible role of climate change, predation on postlarvae and fisheries. Marine Ecology Progress Series 284: 149-167.
Borja A., Franco J. & Pérez V. (2000) A marine biotic index to establish the ecological quality of soft-bottom benthos within European estuarine and coastal environments. Marine Pollution Bulletin 40: 1100-1114.
Bos D., Büttger H., Esselink P., Jager Z., de Jonge V., Kruckenberg H., van Maren B. & Schuchardt B. (2012) De ecologische toestand van het Eems-estuarium en mogelijkheden voor herstel./Der ökologische Zustand des Emsästuars und Möglichkeiten der Sanierung. A&W rapport 1759. Programma Naar Een Rijke Waddenzee/Altenburg & Wymenga, Leeuwarden/Veenwouden.
Buschbaum C., Lackschewitz D. & Reise K. (2012) Nonnative macrobenthos in the Wadden Sea ecosystem. Ocean & Coastal Management 68: 89-101.
Compton T.J., Holthuijsen S., Koolhaas A., Dekinga A., ten Horn J., Smith J., Galama Y., Brugge M., van der Wal D., van der Meer J., van der Veer H.W. & Piersma T. (2013) Distinctly variable mudscapes: Distribution gradients of intertidal macrofauna across the Dutch Wadden Sea. Journal of Sea Research 82: 103-116.
Compton T.J., Bodnar W., Koolhaas A., Dekinga A., Holthuijsen S., ten Horn J., McSweeney N., van Gils J.A. & Piersma T. (2016) Burrowing behavior of a deposit feeding bivalve predicts change in intertidal ecosystem state. Frontiers in Ecology and Evolution 4: 19.
Dankers N., Kühl H. & Wolff W.J. (Eds) (1983) Invertebrates of the Wadden Sea. In: Ecology of the Wadden Sea. Ed. by W.J. Wolff. Balkema, Rotterdam,1, 4/1–4/221.
De Jonge V.N. & Essink K. (1991) Long-term changes in nutrient loads and primary and secondary production in the Dutch Wadden Sea. In: Elliott M, Ducrotoy J-P (eds) Estuaries and Coasts: Spatial and Temporal Intercomparisons. Olsen and Olsen, Fredensborg, p 307-316.
De Jonge V.N., Schuttelaars H.M., van Beusekom J.E.E., Talke S.A. & de Swart H.E. (2014) The influence of channel deepening on the estuarine turbidity levels and dynamics, as exemplified by the Ems estuary. Estuarine, Coastal and Shelf Science 139: 46-59.
De Smet B., Godet L., Fournier J., Desroy N., Jaffré M., Vincx M. & Rabaut M. (2013) Feeding grounds for waders in the Bay of the Mont Saint-Michel (France): the Lanice conchilega reef serves as an oasis in the tidal flats. Marine Biology 160: 751-761.
Dekker, R. & Beukema J.J. (2012) Long-term dynamics and productivity of a successful invader: The first three decades of the bivalve Ensis directus in the western Wadden Sea. Journal of Sea Research 71: 31-40.
Dekker, R. & Drent J. (2013) The macrozoobenthos in the subtidal of the western Dutch Wadden Sea in 2008 and a comparison with 1981-1982. NIOZ-Report 2013-5.
Delefosse M., Banta G.T., Canal-Vergés P., Penha-Lopes G., Quintana C.O., Valdemarsen T. & Kristensen E. (2012) Macrobenthic community response to the Marenzelleria viridis (Polychaeta) invasion of a Danish estuary. Marine Ecology Progress Series 461: 83-94.
Eriksson, B.K., van der Heide T., van de Koppel J., Piersma T., van der Veer H.W. & Olff H. (2010) Major changes in the ecology of the Wadden Sea: human impacts, ecosystem engineering and sediment dynamics. Ecosystems 13: 752-764.
Essink, K. & Dekker R. (2002) General patterns in invasion ecology tested in the Dutch Wadden Sea: the case of a brackish-marine polychaetous worm. Biological Invasions 4: 359-368.
Giere O. & Pfannkuche O. (1982) Biology and ecology of marine Oligochaeta, a review. Oceanography and Marine Biology an Annual Review 20: 173–308.
Gutiérrez J.L., Jones C.G., Strayer D.L. & Iribarne O.O. (2003) Mollusks as ecosystem engineers: the role of shell production in aquatic habitats. Oikos 101: 79-90.
Jensen K.T. (1992) Macrozoobenthos on an intertidal mudflat in the Danish Wadden Sea: comparisons of surveys made in the 1930s, 1940s and 1980s. Helgoländer Meeresuntersuchungen 46: 363-376.
Kraan C., Dekinga A. & Piersma T. (2011) Now an empty mudflat: past and present benthic abundances in the western Dutch Wadden Sea. Helgoland Marine Research 65: 51–58.
Levin L.A., Boesch D.F., Covich A., Dahm C., Erséus C., Ewel K.C., Kneib R.T., Moldenke A., Palmer M.A., Snelgrove P., Strayer D. & Weslawski J.M. (2001) The function of marine critical transition zones and the importance of sediment biodiversity. Ecosystems 4: 430-451.
Lotze H.K. (2005) Radical changes in the Wadden Sea fauna and flora over the last 2000 years. Helgoland Marine Research 59: 71–83.
Möller P. (1986) Physical factors and biological interactions regulating infauna in shallow boreal areas. Marine Ecology Progress Series 30: 33-47.
Nehls G., Diederich S., Thieltges D.W. & Strasser M. (2006) Wadden Sea mussel beds invaded by oysters and slipper limpets: competition or climate control? Helgoland Marine Research 60: 135-143.
Olff H., Alonso D., Berg M.P., Eriksson B.K., Loreau M., Piersma T. & Rooney N. (2009) Parallel ecological networks in ecosystems. Philosophical Transactions of the Royal Society B 364: 1755-1779.
Philippart C,J.M., Beukema J.J., Cadée G.C., Dekker R., Goedhart P.W., van Iperen J.M., Leopold M.F. & Herman P.M.J. (2007) Impacts of nutrient reduction on coastal communities. Ecosystems 10: 95-118.
Polis G.A. & Strong D.R. (1996) Food web complexity and community dynamics. The American Naturalist 147: 813-846.
R Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/.
Reise K. (1982) Long-term changes in the macrobenthic invertebrate fauna of the Wadden Sea - are polychaetes about to take over? Netherlands Journal of Sea Research 16: 29-36.
Reise K. (1998) Pacific oysters invade mussel beds in the European Wadden Sea. Senckenbergiana maritima 28: 167-175.
Reise K., Baptist M., Burbridge P., Dankers N., Fischer L., Flemming B., Oost A.P. & Smit C. (2010) The Wadden Sea – A Universally Outstanding Tidal Wetland. Wadden Sea Ecosystem No. 29. Common Wadden Sea Secretariat, Wilhelmshaven, Germany, page 7 – 24.
Schückel U. & Kröncke I. (2013) Temporal changes in intertidal macrofauna communities over eight decades: A result of eutrophication and climate change. Estuarine, Coastal and Shelf Science 117: 210-218.
Schumacher J., Dolch T. & Reise K. (2014) Transitions in sandflat biota since the 1930s: effects of sea-level rise, eutrophication and biological globalization in the tidal bay Konigshafen, northern Wadden Sea. Helgoland Marine Research 68: 289-298.
Strasser M. (1999) Mya arenaria - an ancient invader of the North Sea coast. Helgoländer Meeresuntersuchungen 52:309-324.
Strasser M., Dekker R., Essink K., Günther C.–P., Jaklin S., Kröncke I., Madsen P.B., Michaelis H. & Vedelh G. (2003) How predictable is high bivalve recruitment in the Wadden Sea after a severe winter? Journal of Sea Research 49: 47–57.
Van Beusekom J.E.E., Bot P.V.M., Carstensen J., Goebel J.H.M., Lenhart H., Pätsch J., Petenati T., Raabe T., Reise K. & Wetsteijn B. (2009) Eutrophication. Thematic Report No. 6. In: Marencic, H. & Vlas, J. de (Eds.), 2009. Quality Status Report 2009. WaddenSea Ecosystem No. 25. Common Wadden Sea Secretariat, Trilateral Monitoring and Assessment Group, Wilhelmshaven, Germany.
Van der Graaf S., de Vlas J., Herlyn M., Voss J., Heyer K. & Drent J. (2009) Macrozoobenthos. Thematic Report No. 10. In: Marencic, H. & Vlas, J. de (Eds), 2009. Quality Status Report 2009. Wadden Sea Ecosystem No. 25. Common Wadden Sea Secretariat, Trilateral Monitoring and Assessment Group, Wilhelmshaven, Germany.
Van der Meer J., Beukema J.J. & Dekker R. (2001) Long-term variability in secondary production of an intertidal bivalve population is primarily a matter of recruitment variability. Journal of Animal Ecology 70: 159–169.
Van der Meer J., Beukema J.J. & Dekker R. (2013) Using stochastic population process models to predict the impact of climate change. Journal of Sea Research 82: 117-121.
Van Loon W.M.G.M., Boon A.R., Gittenberger A., Walvoort D.J.J., Lavaleye M.,
Duineveld G.C.A. & Verschoor A.J. (2015) Application of the Benthic Ecosystem Quality Index 2 to benthos in Dutch transitional and coastal waters. Journal of Sea Research 103: 1–13.
Van Maren D.S., Oost A.P., Wang Z.B. & Vos P.C. (2016) The effect of land reclamations and sediment extraction on the suspended sediment concentration in the Ems Estuary. Marine Geology 376: 147–157.
Van Oevelen D., Soetaert K., Middelburg J.J., Herman P.M.J., Moodley L., Hamels I., Moens T.& Heip C.H.R. (2006) Carbon flows through a benthic food web: Integrating biomass, isotope and tracer data. Journal of Marine Research 64: 453–482.
Verwey, J. (1952) On the ecology of distribution of cockle and mussel in the Dutch Wadden Sea, their role in sedimentation and the source of their food supply. Archives Néerlandaises de Zoologie 10: 171–239.
Voß J., Knaack J. & von Weber M. (2010) Ökologische Zustandsbewertung der deutschen Übergangs- und Küstengewässer 2009. Meeresumwelt Aktuell Nord- und Ostsee 2010/2.
Wood S.N. (2006) Generalized Additive Models: An Introduction with R. Chapman and Hall/CRC.
This report should be cited as: Drent, J., Bijkerk, R., Herlyn, M., Grotjahn, M., Voß, J., Carausu, M.-C., & Thieltges, D. W. (2017). Wadden Sea Quality Status Report: Macrozoobenthos. Common Wadden Sea Secretariat. https://doi.org/10.5281/zenodo.15195273
All 2017 reports may be cited collectively as: Kloepper, S., Baptist, M. J., Bostelmann, A., Busch, J.A., Buschbaum, C., Gutow, L., Janssen, G., Jensen, K., Jørgensen, H.P., de Jong, F., Lüerßen, G., Schwarzer, K., Strempel, R., & Thieltges, D. (2017). Wadden Sea Quality Status Report. Common Wadden Sea Secretariat, Wilhelmshaven, Germany. Downloaded DD.MM.YYYY. qsr.waddensea-worldheritage.org