Photo: Grey seal (Halichoerus grypus). ITAW /Abbo van Neer.
Marine mammals
B. Unger, J. Baltzer, J. Brackmann, S. Brasseur, M. Brügmann, B. Diederichs, A. Galatius, S.C.V. Geelhoed, H. Huus Petersen, L.L. IJsseldijk, T.K. Jensen, A. Jess, D. Nachtsheim, C. Philipp, M. Scheidat, J. Schop, U. Siebert, J. Teilmann, C.B. Thøstesen & A. van Neer
Published 2022
1. Introduction
The Wadden Sea and adjacent North Sea waters were designated as a World Heritage Site due to its “unique, natural and dynamic ecosystem with characteristic biodiversity” (Common Wadden Sea Secretariat, 2010). The World Heritage Site extends from Denmark and Germany to The Netherlands and includes offshore North Sea waters as well as intertidal waters. Several different national and international management and conservation frameworks exist for the special protection of the habitats and inhabitants of this sensitive ecosystem. This requires a coordinated monitoring and management approach. For further information on its extent please check the introduction of the Wadden Sea Quality Status Report. It includes offshore North Sea waters as well as intertidal waters (a.k.a. the Wadden Sea).
The purpose of the Quality Status Report for Marine Mammals is to highlight their current status and developments in the Wadden Sea as well as to suggest improvements in management measures and monitoring approaches. Three marine mammal species occur regularly in the Wadden Sea: the harbour seal (Phoca vitulina), grey seal (Halichoerus grypus) and the harbour porpoise (Phocoena phocoena).
Harbour seal (Phoca vitulina)
The harbour seal (Figure 1) is the most abundant seal species in the Wadden Sea. Recent counts in the Wadden Sea and around Helgoland during the moult surveys in 2021 resulted in a total of 26,721 individuals and 10,902 pups (Galatius et al., 2021). In the past, hunting had drastically reduced the harbour seal population that had dropped to less than 4,000 animals in the mid-20th century. As a result, the states bordering the Wadden Sea implemented hunting bans, in 1962 in The Netherlands, followed by Denmark and Germany, until an area-wide ban was achieved in 1977 (Reijnders, 1981; Reijnders, 1983). Initially, pollution hampered recovery, followed by the occurrence of two Phocine Distemper Virus (PDV) epizootics in 1988 and 2002, reducing the population by 57% and 50%, respectively (Reijnders et al., 1997; Reijnders et al., 2003; Härkönen et al., 2006). After 2002 the population showed a strong recovery and has kept growing since, although with reduced growth rates in the last years (Brasseur et al., 2018; Galatius et al., 2020a).
 Figure 1. Harbour seal (Phoca vitulina). (Photo: ITAW/Abbo van Neer).
Figure 1. Harbour seal (Phoca vitulina). (Photo: ITAW/Abbo van Neer).
Harbour seals haul out on undisturbed sandbanks that are used for breeding, moulting and resting. Breeding takes place from May to August. Having shed their lanugo before birth, pups can swim directly. Pups are weaned after an average of 24 days (Reijnders et al., 2010a; Brasseur, 2017). The peak of the moult in harbour seals is in August. Tagging studies have shown that harbour seals in the Wadden Sea regularly travel tens of kilometres, occasionally more, into the North Sea to forage (Tougaard et al., 2008; Reijnders et al., 2010b; Brasseur, 2017; Vance et al., 2021). They mainly perform dives to the seafloor which in the southern North Sea is limited to depths of 50 m. Dives generally last 3-4 minutes (Wilson et al., 2015; Vance et al., 2021). The diet varies locally and between seasons but includes benthic fish species such as gadoids, flatfish and sand eels (Gilles et al., 2008; de la Vega et al., 2016; Aarts et al., 2019; Wilson & Hammond, 2019).
Grey seal (Halichoerus grypus)
Historically, grey seals (Figure 2) were abundant in the Wadden Sea (Prummel & Heinrich, 2005). However, by the 16th century, the species had completely disappeared from the area probably due to severe hunting (Härkönen et al., 2007). The species gradually returned in the mid-20th century, forming a small breeding colony in the German Wadden Sea and later in the Dutch part of the Wadden Sea (Reijnders et al., 1995). Since their return, the grey seal numbers have constantly grown. During the first coordinated survey in 2006 (including only Germany and the Netherlands) a total of 2,139 grey seals were counted (Reijnders et al., 2006). By 2021 their numbers had grown to 9,096 seals (Brasseur et al., 2021). In addition to an increase in numbers, the distribution of grey seals in the Wadden Sea has expanded to the Danish waters. Therefore, the surveys also include the Danish Wadden Sea since 2015 (Jensen et al., 2015). Demographic modelling has shown that a proportion of the grey seals in the Wadden Sea observed during the moult surveys returns to the British Isles to breed, where the population is estimated to comprise 152,800 animals (SCOS, 2019). But there is also an influx of grey seals from the UK into the Wadden Sea fuelling the breeding population (Brasseur et al., 2015). For management of the population, the entire North Sea is therefore considered one assessment unit (OSPAR).
 Figure 2. Grey seal (Halichoerus grypus). (Photo:ITAW /Abbo van Neer).
Figure 2. Grey seal (Halichoerus grypus). (Photo:ITAW /Abbo van Neer).
Grey seals generally require high sandbanks above the intertidal zone in the Wadden Sea for breeding and moulting. Breeding takes place during winter (mainly between November-December depending on the area). The pup is born with white fur (lanugo) which protects it from cold air temperatures. The pups are weaned after approximately 19 days and stay on land at least until their first moult around 3-6 weeks after birth, acquiring the typical patchy grey adult fur. Moult peak of the adults in the Wadden Sea occurs between March and April.
Grey seals feed mainly on demersal fish species and their diet largely overlaps with that of the harbour seals (Wilson & Hammond, 2019; Damseaux et al., 2020). In comparison to harbour seals, grey seals from the Wadden Sea Area may cover larger distances (McConnell et al., 1999; Jones et al., 2015), even as pups (Peschko et al., 2020). Adult grey seals dive between 4 to 10 minutes to a depth of up to 200 m (Perrin et al., 2009).
Recent studies have shown that grey seals also prey on harbour seals as well as other grey seals (van Neer et al., 2021) and harbour porpoises (Bouveroux et al., 2014; Leopold, 2015; van Neer et al., 2020).
Harbour Porpoise (Phocoena phocoena)
The harbour porpoise (Figure 3) is the most abundant cetacean species in the North Sea (Hammond et al., 2013; Hammond et al., 2021). Parts of the Wadden Sea Area show high summer densities and calf occurrence, leading to the designation of the Whale Sanctuary in the north of the World Heritage Site. It is estimated that the North Sea population accounts for 345,000 animals (Hammond et al., 2017).
The distribution of harbour porpoises is thought to be mostly driven by the availability of prey species, such as gobies, herring and sand eel (Gilles et al., 2009; Leopold et al., 2015b; Gilles et al., 2016). However, anthropogenic disturbances are known to influence harbour porpoise distribution and abundance (see sub-section “Threats to marine mammals in the Wadden Sea” for more detailed information).
 Figure 3 Harbour porpoise (Phocoena phocoena). (Photo: ITAW /Abbo van Neer).
Figure 3 Harbour porpoise (Phocoena phocoena). (Photo: ITAW /Abbo van Neer).
The birth period of harbour porpoises in the North Sea peaks in June and July, and females usually give birth to one calf (Sørensen & Kinze, 1994; Hasselmeier et al., 2004).
2. Status and trends
Overall trends
Harbour seal (Phoca vitulina)
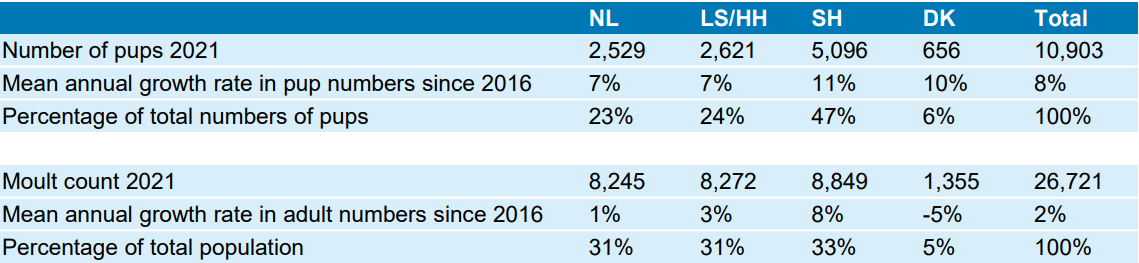
In June 2021, a total of 10,902 pups were counted in the Wadden Sea, constituting an increase of 10% relative to the count in 2020 (9,954 pups). In the same year, 26,721 harbour seals were counted during the moult (Table 1, Figure 4). Since 2012, the harbour seal population seems to have stabilized and is estimated at around 40,000 animals with an average annual growth of 1% per year (Galatius et al., 2021), and thus much lower than the growth rate of 8.7% estimate for 2003-2014 (Brasseur et al., 2018). As a comparison, the long term trend for the pup counts has shown an annual increase of on average 5% between 2012 and 2021 and an average annual increase of 8.9% between 2003 and 2014 (Figure 5) (Brasseur et al., 2018). It is paradoxical that the growing annual pup counts comprising of up to 10,000 animals do not result in population growth while neither migration to other populations nor increased mortality is observed.
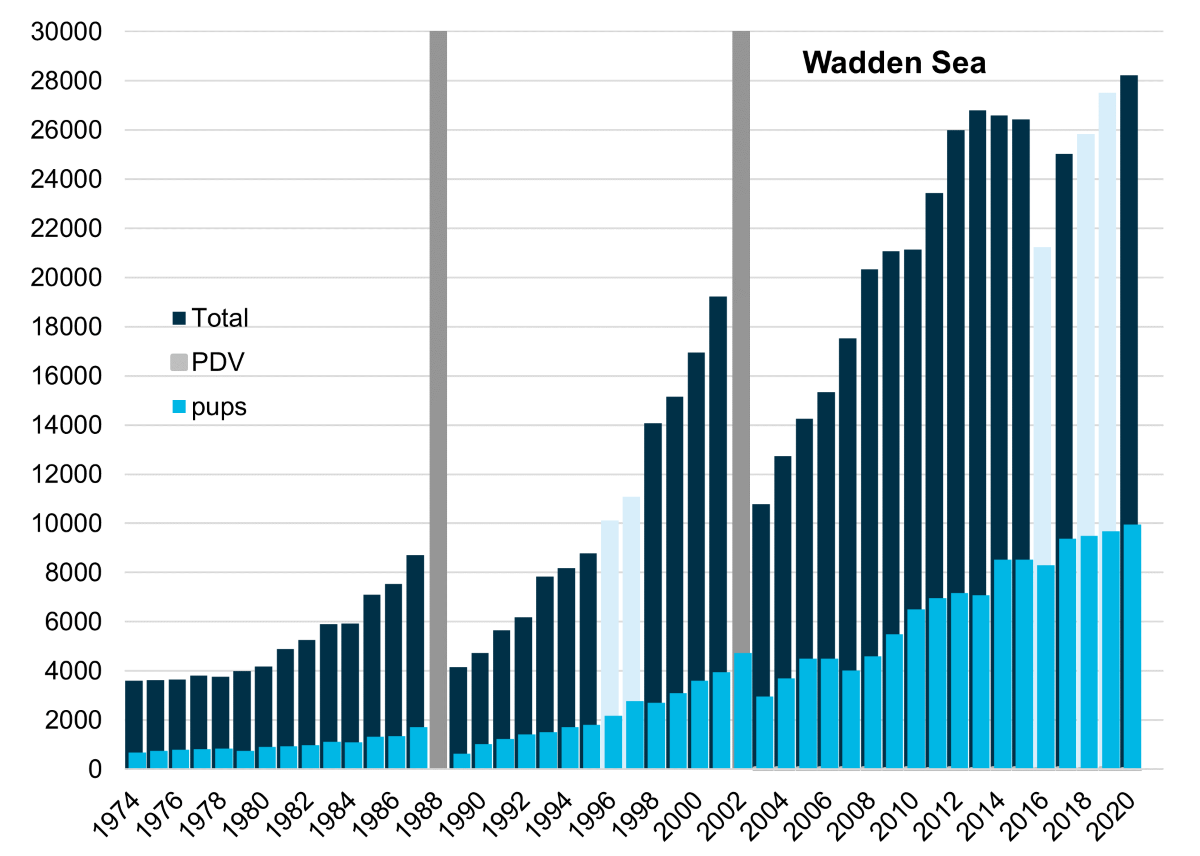 Figure 4. The total number of harbour seals counted in the Wadden Sea. The dark blue bars represent the total number of harbour seals during moult (light when counts were incomplete) and the lighter blue bars represent the number of pups. Grey bars indicate numbers counted during outbreaks of the Phocine Distemper Virus (PDV).
Figure 4. The total number of harbour seals counted in the Wadden Sea. The dark blue bars represent the total number of harbour seals during moult (light when counts were incomplete) and the lighter blue bars represent the number of pups. Grey bars indicate numbers counted during outbreaks of the Phocine Distemper Virus (PDV).
Table 1. Overview of the harbour seal pups and moult counts per region in the Wadden Sea based on the numbers published in the annual CWSS report. NL = The Netherlands, LS/HH = Lower Saxony & Hamburg; SH = Schleswig-Holstein (Wadden Sea); DK = Denmark.
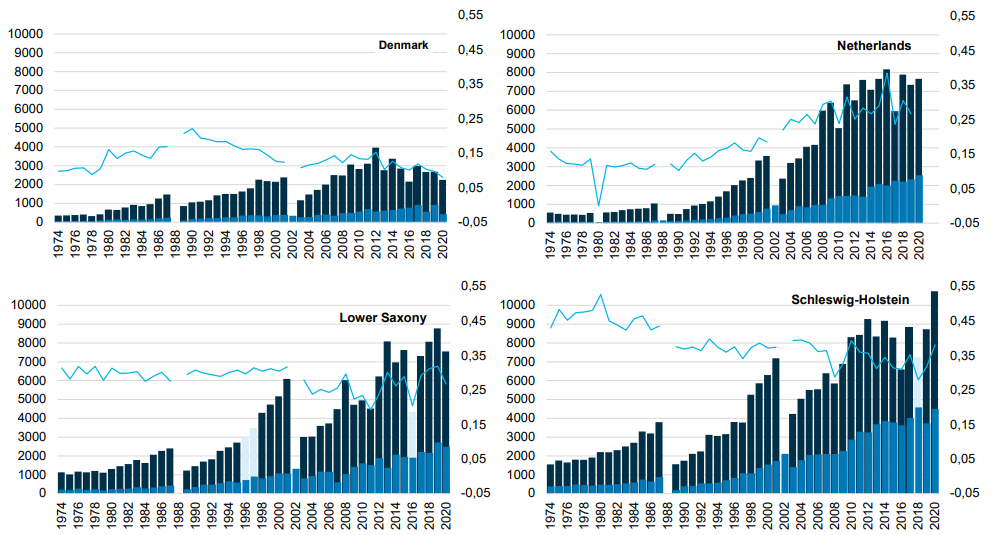 Figure 5. The number of harbour seals counted in the different Wadden Sea areas. The dark blue bars represent the number of harbour seals during moult (light blue when counts were incomplete) and the lighter blue bars represent the number of pups. The light blue line indicates the relative importance of an area for the population (the numbers in the area compared to the total number in the Wadden Sea).
Figure 5. The number of harbour seals counted in the different Wadden Sea areas. The dark blue bars represent the number of harbour seals during moult (light blue when counts were incomplete) and the lighter blue bars represent the number of pups. The light blue line indicates the relative importance of an area for the population (the numbers in the area compared to the total number in the Wadden Sea).
Regional trends
Harbour seals in Denmark
The harbour seal moult count in Denmark was approximately halved by the PDV outbreak in 2002, from 2,150 to 1,150. After this crash, the counts grew by an annual average rate of 12% to around 4,000 in 2012. However, since then the trend has reversed and the Danish counts have decreased by an annual 5% to around 1,355 in 2021. The latest count constitutes 5% of the Wadden Sea total, a decrease from an average of 14% over the study period. In contrast, the numbers of pups counted since 2012 showed no trend. The annual mean number for the area is 650, and in recent years it has shown large fluctuations from 429 to 919 pups. The lack of a trend in the pup counts and decrease in moult counts reflect the general tendency in the Wadden Sea Area in which increasing numbers of pups are observed relative to the moult counts. The distribution of harbour seals in the Danish Wadden Sea has been stable over the last 10 years. Most moulting seals and most pups are counted in the central drainage areas between Rømø and Mandø (mean 34% moulting seals and 31% pups) and Mandø and Fanø (36% moult and 40% pups), while fewer seals are counted in the far north and south. There has, however, been a tendency that fewer pups are counted on the sandbanks that are most used by tourist operators.
Harbour seals in Germany
Federal State of Schleswig-Holstein, including Helgoland
Similar to the Dutch moult counts, the harbour seal counts in the Schleswig-Holstein Wadden Sea showed an increase after the PDV outbreak in 2002 from 4,235 individuals counted in 2003 up to 8,309 in 2010, but with an average annual rate of 10% growth, it was less strong than in the Dutch Wadden Sea. From 2010 onwards, the moult counts showed no clear trend but a high variability with an average annual increase of 4% until 2021. Because of the avian influenza outbreak, higher numbers of dead harbour seals were found along the shore of Schleswig-Holstein in winter 2014/15, and in 2015, the moult count was reduced by 10% compared to 2014. Remarkably, the numbers showed an abrupt rise of about 23 % from 2019 to 2020, with 10,746 harbour seals counted, followed by a decrease of 18% from 2020 to 2021. On the island of Helgoland, the trilateral monitoring started in 2018 because of rising harbour seal numbers. In 2021, 117 harbour seals have been counted on the island during the moult survey.
From 2003 to 2021, the pup counts in the Schleswig-Holstein Wadden Sea have more than tripled from 1,407 up to 5,096 in 2021. Over this entire period, about 45% of the pups in the Wadden Sea were born in Schleswig-Holstein, with little fluctuation of the importance of the area. The fraction of the Wadden Sea harbour seal population counted during the moult in Schleswig-Holstein has decreased slightly from 39% in 2003 to 33% in 2021.
Federal State of Lower Saxony and Hamburg
The abundance of harbour seals in the Lower Saxony and Hamburg Wadden Sea has stabilised in recent years. The observed trend over the last 20 years is comparable to the Netherlands and Schleswig-Holstein. From 2015 to 2020, the moult counts remained relatively constant between a minimum of 7,311 counted in 2017 and a maximum of 8,772 animals counted in 2019. Between 2020 and 2021, there was a 10% increase in the moult count following a decrease of 14% in the previous year.
The losses caused by the 2014/ 2015 influenza epidemic (H10N7) were nowhere near the numbers seen in Schleswig-Holstein. This was reflected not only in the number of dead animals found in winter 2014/ 2015 but also in the moult counts in 2015, which were about 9 % higher than in 2014.
The importance of Lower Saxony and Hamburg as a pupping area is high over the entire observation period. Similar to the pup growth in the Netherlands (11%), the number of pups increased on average by 9% per year between 2004 and 2021. In 2020, a particularly high proportion of pups compared to the moult counts was observed (33%). In June 2021, 2,621 pups were counted, representing approximately 24% of all harbour seal pups in the Wadden Sea. The proportion of pups is slightly higher in the eastern part of this census area.
Harbour seals in the Netherlands
By 2011, moult counts in the Dutch Wadden Sea had tripled compared to the numbers after the PDV outbreak in 2002. During this period, counts grew by approximately 17% annually. However, from 2011 onwards, annual growth dropped to about 1% and numbers currently fluctuate around 7,500 animals. Surprisingly, this sudden change in trend is not reflected in the pup counts, for which the growth rate has slowed down slightly, but still showed rates of more than 7% annually over the same period. In 2021, more than 2,500 pups were counted throughout the Dutch Wadden Sea.
The small number of seals that may have moved to the Dutch Delta, where growth is approximately 200 seals per year, does not explain the lack of growth in the Wadden Sea (https://www.clo.nl/indicatoren/nl). Moreover, the numbers observed in the Delta are potentially also influenced by animals visiting from France and the UK as tracked and tagged seals show some exchange (Galatius et al., 2020b). The interpretation of future monitoring data from the Wadden Sea might benefit from also considering changes in the Delta and these neighbouring colonies.
Over the almost 50 years of trilateral harbour seals monitoring, the Dutch area has become increasingly important for the Wadden Sea harbour seal population: in the 1970s and ’80s, the Dutch Wadden sea was harbouring only about 12% of the total Wadden Sea population. This distribution has shifted in the past decade and currently, 25-30% of all Wadden Sea harbour seals are using the Dutch Wadden Sea during the moult. This shift is also reflected in the pup counts; currently, well over 20% of the pups are born in the Netherlands, while this was only 7-8% in the 1970s and ’80s (Figure 5).
Grey Seals (Halichoerus grypus)
After having been absent for several centuries, grey seals started to recolonise the Wadden Sea around the middle of the last century. Compared to the observations in the UK, where the seals seem to have originated from, the grey seals in the Wadden Sea typically breed late in the year: in November-January and moult in March-April.
The local grey seal breeding colonies, indicated by the peak of pups born, have grown at an average annual rate of 11% over the past five years (Brasseur et al., 2021). In 2021, 1,927 grey seal pups were counted in the area, comprising of the Wadden Sea and Helgoland. The majority (>50%) of the grey seal pups were born in the Dutch part of the Wadden Sea (Table 2).
Table 2. Overview of the grey seal pups and moult counts per region in the Wadden Sea based on the numbers published in the annual CWSS report. NL = The Netherlands; LS / HH = Lower Saxony & Hamburg; SH = Schleswig-Holstein (Wadden Sea); Hel = Helgoland; DK = Denmark.
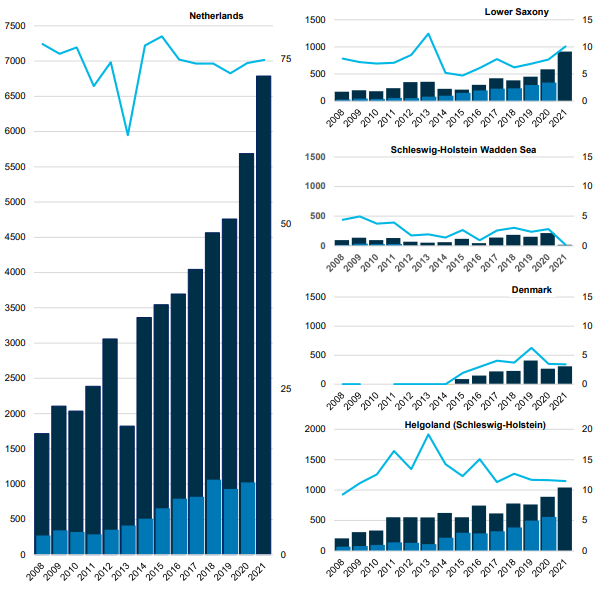
In 2021 a total of 9,069 grey seals were counted during moult. These numbers have grown at an average annual rate of 13% over the past five years. The seals observed in this period are a mixture of animals breeding locally and animals from adjacent areas, mainly the UK (Brasseur et al., 2015). This might explain the distribution of grey seals throughout the Wadden Sea (Table 2). Overall, it seems that the numbers of grey seals in the area are continuing to grow, and expand towards the east (Figure 6, Figure 7).
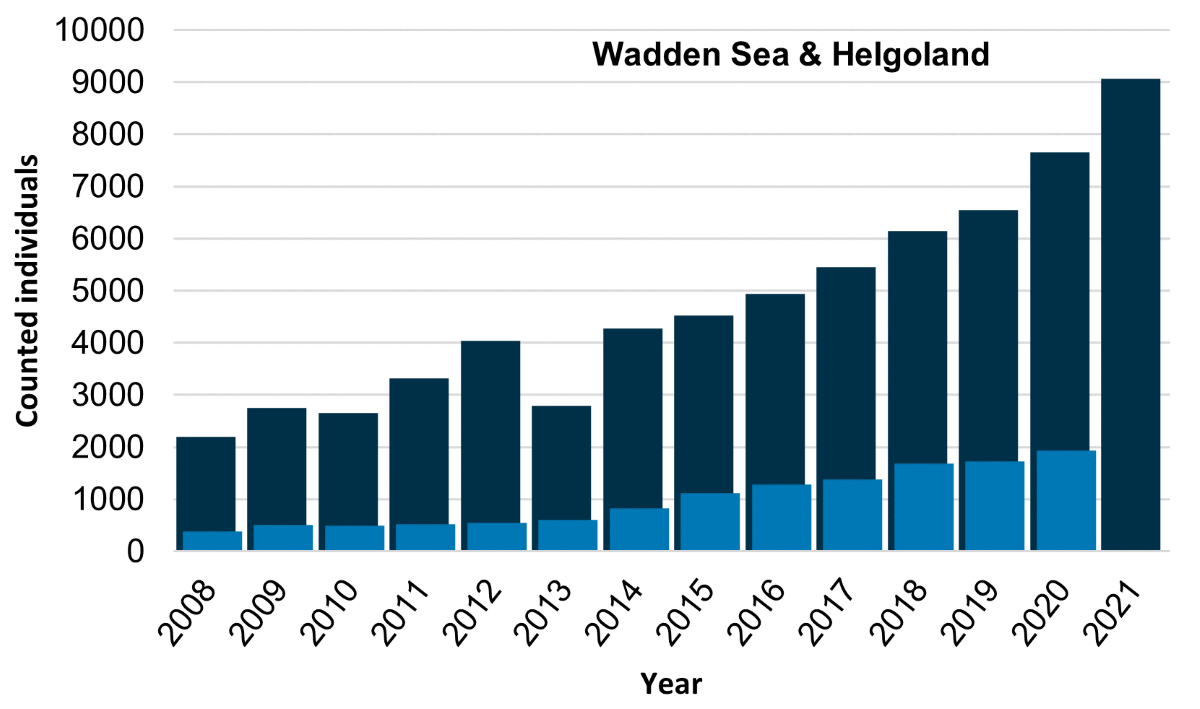 Figure 6. The number of grey seals counted during grey seal moult counts in the Wadden Sea Area between 2008 and 2021 (dark bars) and the number of pups counted in the preceding pupping season (light bars).
Figure 6. The number of grey seals counted during grey seal moult counts in the Wadden Sea Area between 2008 and 2021 (dark bars) and the number of pups counted in the preceding pupping season (light bars).
Figure 7. The number of grey seals counted in the different Wadden Sea Areas. The bars number of the total number of grey seals during moult (dark blue) and the number of pups (light blue). The light blue line indicates the relative importance of the numbers in the area compared to the total number of grey seals counted during the moult in March-April.
Grey seals in Denmark
Denmark was the last area to experience a large influx of grey seals and consequently, monitoring of grey seal moulting and pupping in Denmark only began in 2014 for pupping and 2015 for moulting. Before grey seals were regularly observed in summer during the harbour seal counts (<100 individuals). In 2015, 88 seals were counted during the moult, and this number has steadily increased, with 309 counted in 2021 (Figure 7). The counts show an annual growth rate of 25%. Grey seal pups are still few in Denmark. On the synchronised trilateral count dates, only one pup has been observed in 2015. However, in most years, at least one pup has been observed later in the season in January, suggesting that the few births in Denmark are timed later than in the western parts of the Wadden Sea. In the first years of observations (2015-2017), many grey seals, as well as the few pups, were observed at Rømø Flak, northwest of Rømø. However, this sandbank is decreasing in size and its propensity for flooding is increasing. Since 2018, the majority of the grey seals has been observed at Knudedyb, northwest of Mandø, the two pups observed in 2018 and 2020 were also found on this large and high sandbank.
Grey seals in Germany
Federal State of Schleswig-Holstein, including Helgoland
Grey seals are found in two different areas in Schleswig-Holstein, in the Wadden Sea National Park and the offshore island Helgoland. Each of these areas shows a different development of the local grey seal stock since 2008.
The oldest grey seal colony in the Wadden Sea of Schleswig-Holstein showed a strong increase in pup counts from 12 pups in 2008 to 40 pups in 2010 (Figure 7). Following some severe storms, the main haul-out, Jungnamensand close to Amrum, has eroded significantly and the frequency of flooding increased since 2010. The area became less suitable for pupping and pup counts decreased and none were counted in 2020. Still, the numbers during moult in the Wadden Sea are growing slowly. Distribution has shifted, with grey seals switching between different sandbanks in the region depending on the actual water level. The number of grey seals counted in this area has grown from 98 in 2008 to 218 in 2020.
The development of the pup counts on Helgoland differs considerably from the Schleswig-Holstein Wadden Sea. Since the beginning of the trilateral monitoring, they show a strong increase from 52 pups in 2008 to 559 pups in 2021, representing an average increase of 23% per year. Similarly, an increase was observed during moult on Helgoland: from 2008 the colony size increased from 206 to 1,041 grey seals in 2021, representing an average increase of 15% per year.
Federal State of Lower Saxony and Hamburg
In Lower Saxony and Hamburg, the number of grey seals, both animals during the moulting season of the grey seals and pups, have been increasing (Figure 7). During one of the first years of the coordinated monitoring in 2008 only 174 adult animals were counted during the moult. This number has since been growing to 913 individuals in 2021, representing an average growth of 24 % per year.
This local trend is observed in the number of pups during winter counts which also show an average increase of 24 % per year between winter 2006/2007 (15 pups) and 2020/2021 (341 pups).
Beside this increase in abundance, the grey seals also show a change in distribution in the last years, slowly expanding their range. Initially, the majority of animals was counted on the Kachelotplate, a sandbank in the west close to the Dutch border, with only few sightings outside of this area. While the majority of grey seals is still observed on the Kachelotplate, growing numbers are now reported from several sandbanks in the east of the Wadden Sea of Lower Saxony. This change in distribution is an indication of the positive trend of grey seals in the Wadden Sea of Lower Saxony and Hamburg.
Grey seals in the Netherlands
The number of grey seal pups observed in the Netherland has fluctuated in the past 3 years, and in 2020 slightly over 1,000 pups were counted (Figure 7). This could indicate that the number of breeding grey seals in the Dutch Wadden Sea is not following the growth rate observed in the whole Wadden Sea. More than 90% of pupping is concentrated on one site (the sandbank Richel between the islands of Vlieland and Terschelling) though some breeding is observed both to the west and east of this site. Regularly, especially after storms, pups are observed on the inhabited islands, where the grey seal mother sometimes continues to suckle her pup. During the moult surveys, the number of grey seals in the Dutch Wadden Sea continues to grow and has increased by 19% annually since 2017, amounting to 6,788 seals in 2021 (Figure 7). Evidently, the number of grey seals observed during the moult is strongly influenced by an annual influx of migratory animals from the UK (Brasseur et al., 2015). The influx of animals visiting is also apparent in the Dutch Delta area, where numbers have grown at an average rate of 16% per year to well over 1,500 animals in 2020, despite the almost complete lack of pups born in the region (https://www.clo.nl). These numbers are currently not included in the Wadden Sea surveys, but tracking data show regular exchange between the areas, and the numbers in neighbouring colonies therefore should be considered when evaluating the status of the Wadden Sea colonies (similar to Helgoland).
Exchange within the Wadden Sea and between the Wadden Sea and other areas
Being dependent on land to breed, moult and rest, harbour and grey seals tend to commute between offshore areas and specific sandbanks to haul out periodically after feeding at sea. In many ways, the seals can therefore be considered central place foragers and yet, are seen as non-migratory (Orians & Pearson, 1979; Härkönen & Harding, 2001; Russell et al., 2015). However, the seals may occasionally switch away from preferred haul-out sites or foraging areas, transferring over large distances to different haul-outs and feeding grounds. Understanding these exchanges of seals in the Wadden Sea and their fluctuating dependency on the different areas is key to managing the populations and ensuring their long-term protection.
Most telemetry data from the past decades have demonstrated how seals regularly leave the Wadden Sea to feed during trips of up to multiple days, sometimes travelling hundreds of kilometres offshore in the North Sea, returning to the same haul-out sites (Tougaard et al., 2008; Dietz et al., 2013; Brasseur, 2017; Vance et al., 2021).
In some cases, this pattern changes for example during the breeding season, when seals that have been foraging in one region return to specific breeding sites in other regions, i.e. philopatry or site fidelity (Greenwood, 1980). This has been observed in female harbour seals from Dutch waters that migrated up to 300 km to give birth, and in female grey seals from the Wadden Sea migrating to the UK to breed (Brasseur et al., 2015; Brasseur, 2017; Brasseur et al., 2018). Exchanges over longer distances during the moult are also observed as for example the estimated grey seal numbers during the moult generally exceed estimates based on breeding results (Brasseur et al., 2015).
Moreover, especially young seals, inexperienced in utilising the available haul-out sites, in an effort to find sufficient food and explore possible new territories, may display movements at a very large scale (Ailsa et al., 2001; Brasseur, 2017). This has been demonstrated in newly weaned grey seal pups from Helgoland that performed trips throughout the North Sea and beyond within their first year of life (Peschko et al. (2020); ITAW, unpublished data; Figure 8). They visited nearly all states bordering the North Sea as well as Sweden and used haul-out sites in most of these states utilising on average an area of 8425 km²+/-4945 km² (median +/-sd, n=14; ITAW, unpublished data). Long-term tracking of animals or a comprehensive photo ID programme will be needed to demonstrate if and how these animals return after years to the Wadden Sea Area to breed.
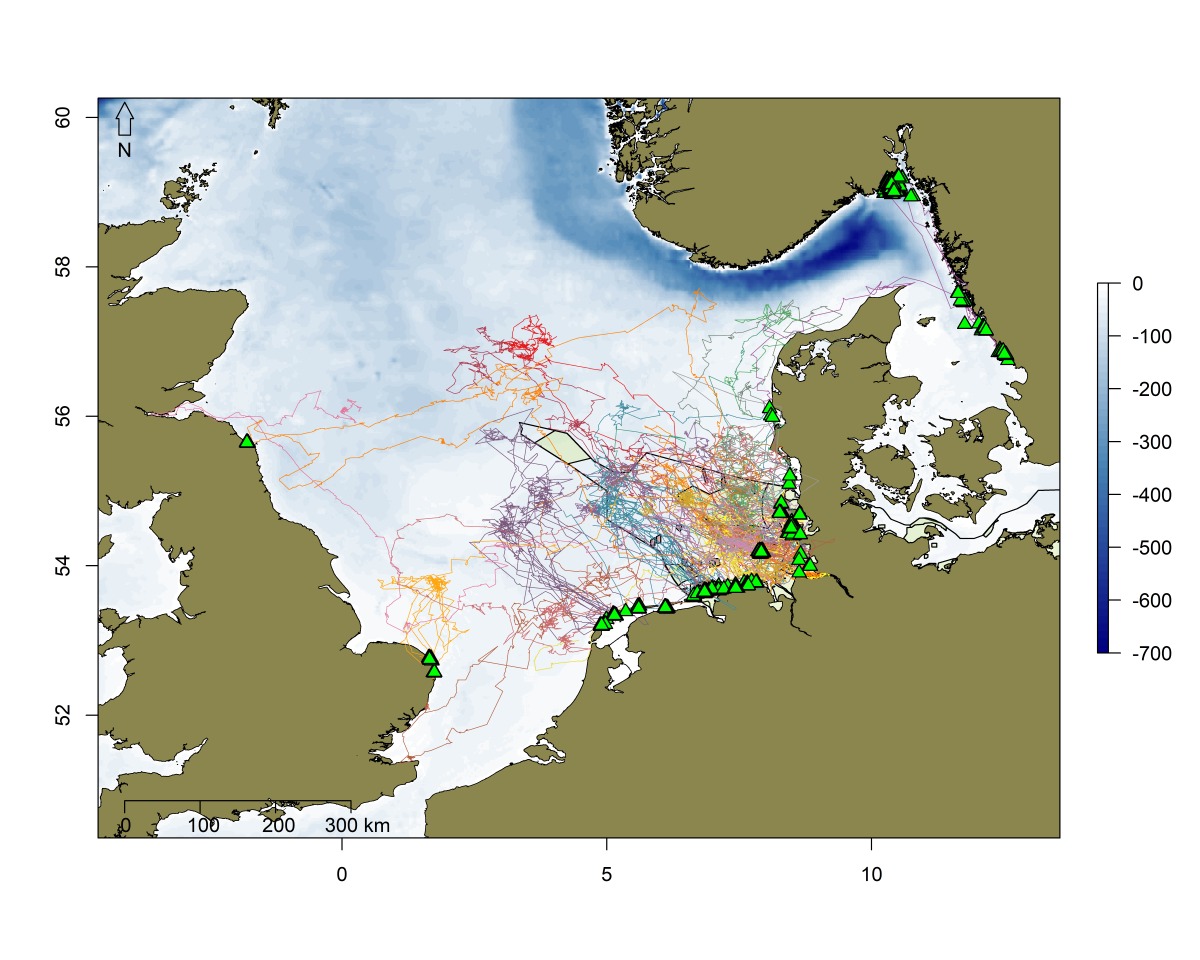 Figure 8. Tracks of juvenile grey seals tagged within the first weeks after weaning on Helgoland, Germany (n=27). Green triangles represent haul out events. Tracks were generated from filtered ARGOS positions (Photo: ITAW, unpublished data).
Figure 8. Tracks of juvenile grey seals tagged within the first weeks after weaning on Helgoland, Germany (n=27). Green triangles represent haul out events. Tracks were generated from filtered ARGOS positions (Photo: ITAW, unpublished data).
Although regarded as non-migratory central place foragers, data strongly indicate that substantial parts of the Wadden Sea seals are dependent on their possibilities to move throughout the Wadden Sea Area as well as between the Wadden Sea and surrounding regions. In light of the planned human development within the North Sea, this knowledge and the understanding of underlying mechanisms must be further developed and included in management and impact assessments to ensure the long-term protection of these key species.
Harbour porpoise (Phocoena phocoena)
Aerial surveys
The distribution and abundance of harbour porpoises are typically assessed by line-transect distance sampling during aerial or shipboard surveys (Scheidat et al., 2008; Gilles et al., 2009; Hammond et al., 2013). To provide unbiased abundance estimates surveys follow transects that are designed to allow a known coverage probability for the area (Buckland et al., 2001). During data collection, standardized protocols are used, based on the SCANS surveys method (Hammond et al., 2002; Hammond et al., 2013). The national monitoring programmes in the Netherlands, Germany and Denmark use aerial surveys to cover the respective territorial waters (Exclusive Economic Zones) and/or focus on specific areas, such as Natura2000 sites (Gilles et al., 2009; Scheidat et al., 2012; Sveegaard et al., 2019). Monitoring programmes in Germany and Denmark also cover parts of the Wadden Sea World Heritage Site but are difficult to conduct in intertidal areas. During low tide, animals can only occur in narrow tidal channels not necessarily covered by the transects (Figure 9, Figure 10). In addition, the often turbid waters make animals below the surface less visible from the plane, thus reducing the sighting probability. This makes the method less effective to estimate density and abundance or investigating habitat use in the Wadden Sea.
The harbour porpoises from the Wadden Sea are considered to be part of the wider North Sea population. Three assessments of the abundance of harbour porpoises in the North Sea have been made in the frame of SCANS, SCANS-II and SCANS-III (Hammond et al., 2002; Hammond et al., 2013; Hammond et al., 2017). The latest survey from 2016 provided an estimate of 345,373 harbour porpoises (95% confidence interval: 246,526 - 495,752) in the North Sea. Based on these three large-scale surveys, there is evidence that the distribution of harbour porpoises has changed over the years. A notable southward shift was detected with increasing porpoise densities in the south-western North Sea (Hammond et al., 2013; Hammond et al., 2017). Nevertheless, the abundance of harbour porpoises in the entire North Sea is within the same range as previous surveys (Hammond et al., 2017).
With higher temporal and spatial resolution, national aerial survey monitoring programmes are able to give better insights into the fine-scale distribution of harbour porpoises and reveal changes in abundance. A recent study estimated trends in absolute harbour porpoise abundance in the German North Sea based on aerial surveys conducted between 2002–2019 (Nachtsheim et al., 2021). Over the years, harbour porpoise abundance in northern areas (e.g. the Natura2000 site “Sylt Outer Reef” west of Sylt) has shown a decreasing trend, whereas an increase in harbour porpoise abundance was detected in more southern areas (e.g. between Borkum and the Elbe estuary). Overall, the trend for the entire German North Sea was negative with a median decline of -1.79% per year (Nachtsheim et al., 2021). These changes could indicate a further southwest shift of the North Sea population, as shown by the large-scale surveys (Hammond et al., 2017). However, the underlying drivers of the observed trend are still unknown and deserve further investigation.
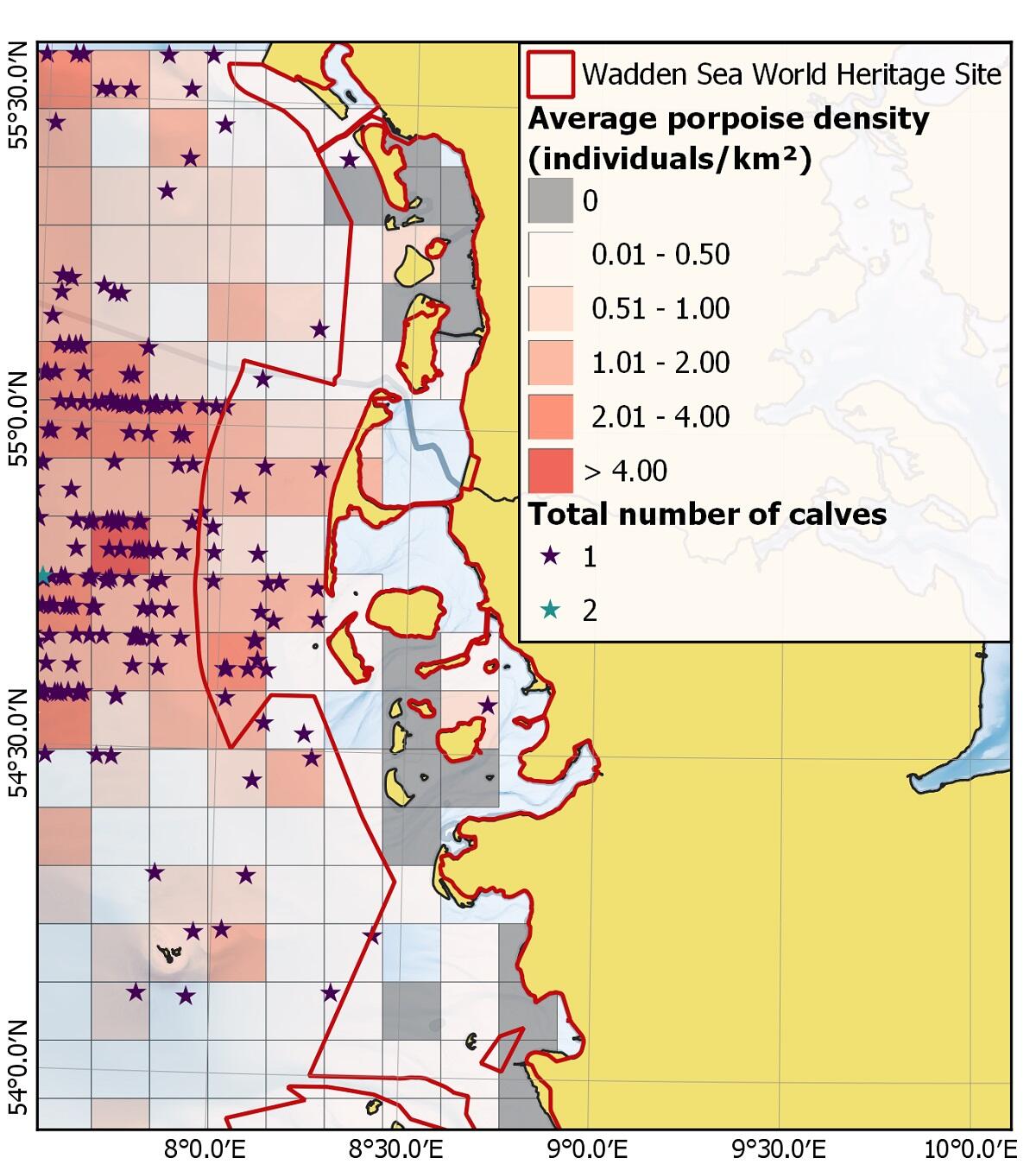 Figure 9. Average porpoise density and calf sightings for Danish (2011-2019) and German waters (2002 to 2020) during summer (June, July, August) (adapted from Scheidat et al. (under review)).
Figure 9. Average porpoise density and calf sightings for Danish (2011-2019) and German waters (2002 to 2020) during summer (June, July, August) (adapted from Scheidat et al. (under review)).
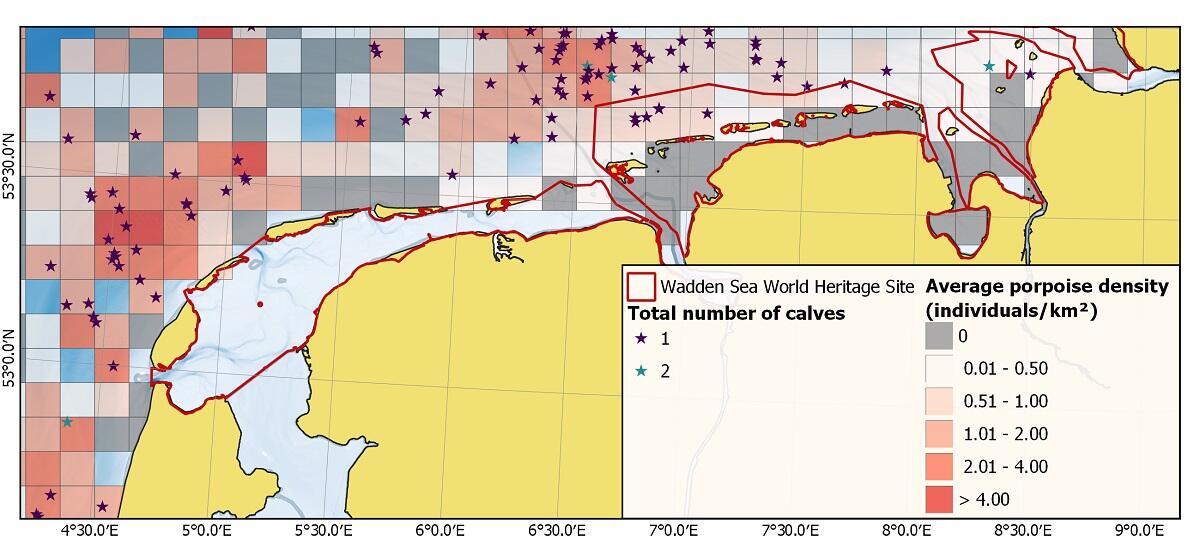 Figure 10. Average porpoise density and calf sightings for German (2002 to 2020) and Dutch waters (2008 to 2020) during summer (June, July, August) (adapted from Scheidat et al. (under review)).
Figure 10. Average porpoise density and calf sightings for German (2002 to 2020) and Dutch waters (2008 to 2020) during summer (June, July, August) (adapted from Scheidat et al. (under review)).
Passive Acoustic Monitoring (PAM)
The use of PAM allows the continuous monitoring of harbour porpoise occurrence and behaviour. Six PAM stations equipped with echolocation click detectors (C-PODs, Chelonia Ltd) were deployed in the German Wadden Sea Area within the framework of the Natura 2000 monitoring for harbour porpoises (Baltzer et al., 2018). Two stations in Lower Saxony collected data from 2011 until 2019, whereas data have been collected and evaluated at four monitoring stations in Schleswig-Holstein in the period 2011-2020. These stations were located in the outer Wadden Sea (Westerland and Rochelsteert), and in the inner waters (Lister Tief and Meldorfer Bucht) (Figure 11).
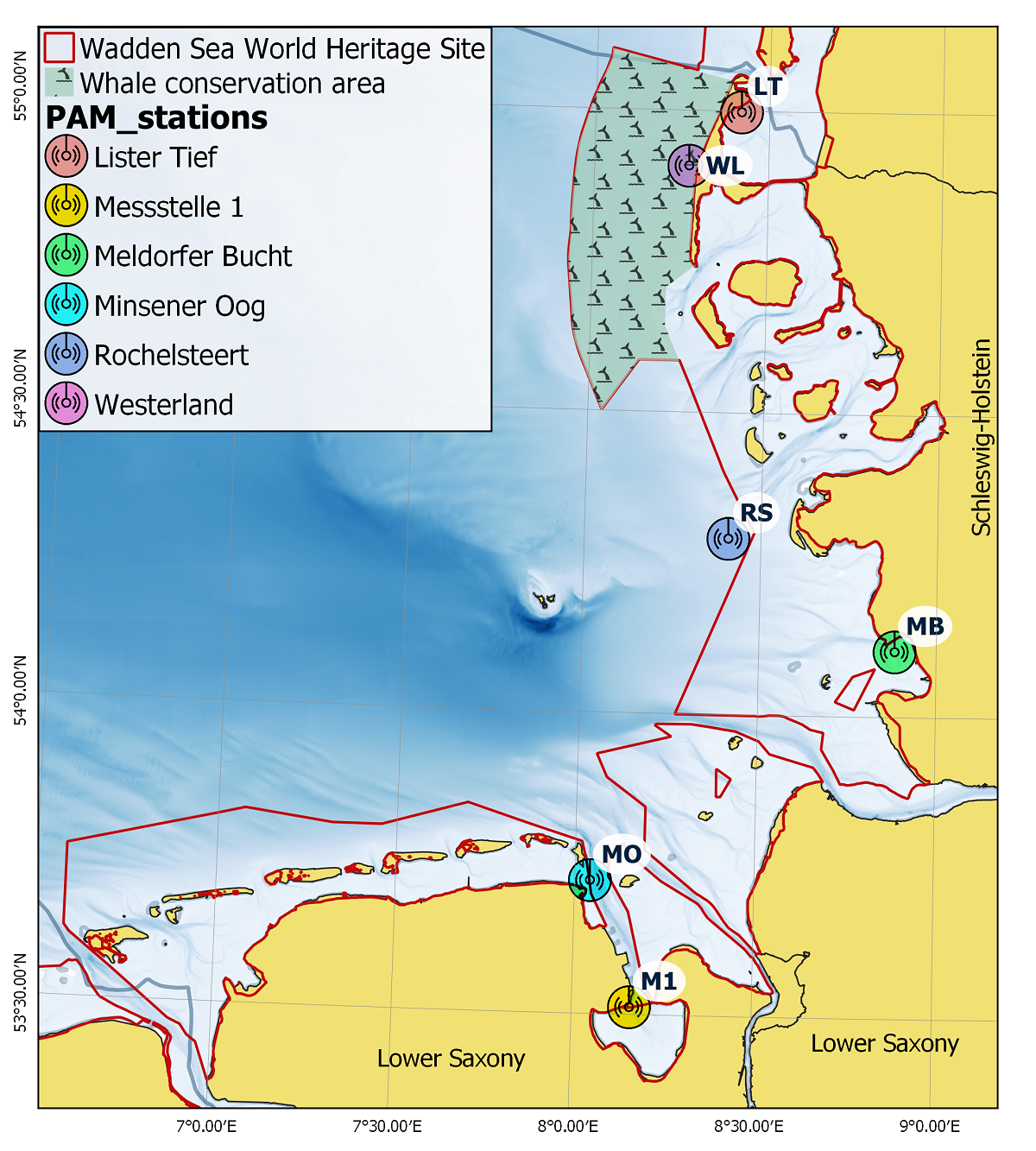 Figure 11. Positions of the PAM stations in the German part of the Wadden Sea World Heritage Site (adapted from Scheidat et al. (under review)).
Figure 11. Positions of the PAM stations in the German part of the Wadden Sea World Heritage Site (adapted from Scheidat et al. (under review)).
Harbour porpoise relative occurrence was expressed as the percentage of how many 10-minute detection intervals contained porpoise clicks per day (DP10min/day). Detection rates showed spatial and temporal differences between the four stations in Schleswig-Holstein. The lowest detection rates occurred at the northernmost station Lister Tief with < 5% DP10min/day while Westerland station had the highest detection rates (up to 20%). Yearly detection rates showed no trend in 2011-2020. A GAM analysis provided insight into seasonal patterns and the influence of time of day and tide on porpoise click occurrence (Baltzer et al., 2018). In Figure 12 the detection probability of porpoises being present is represented relative to the y-axis, with any values y>1 indicating a higher likelihood of porpoises being detected, and values y<1 a lower likelihood of porpoises being detected. All stations revealed a peak in spring (mid-March - mid-April) (Figure 12), but the seasonal pattern outside this period differed per station. Time of day had only a minor influence on detection rates but for some stations a tidal effect could be observed during high tide or in the period between high and low tide. The data show that there was high variability between stations (Zein et al., 2019).
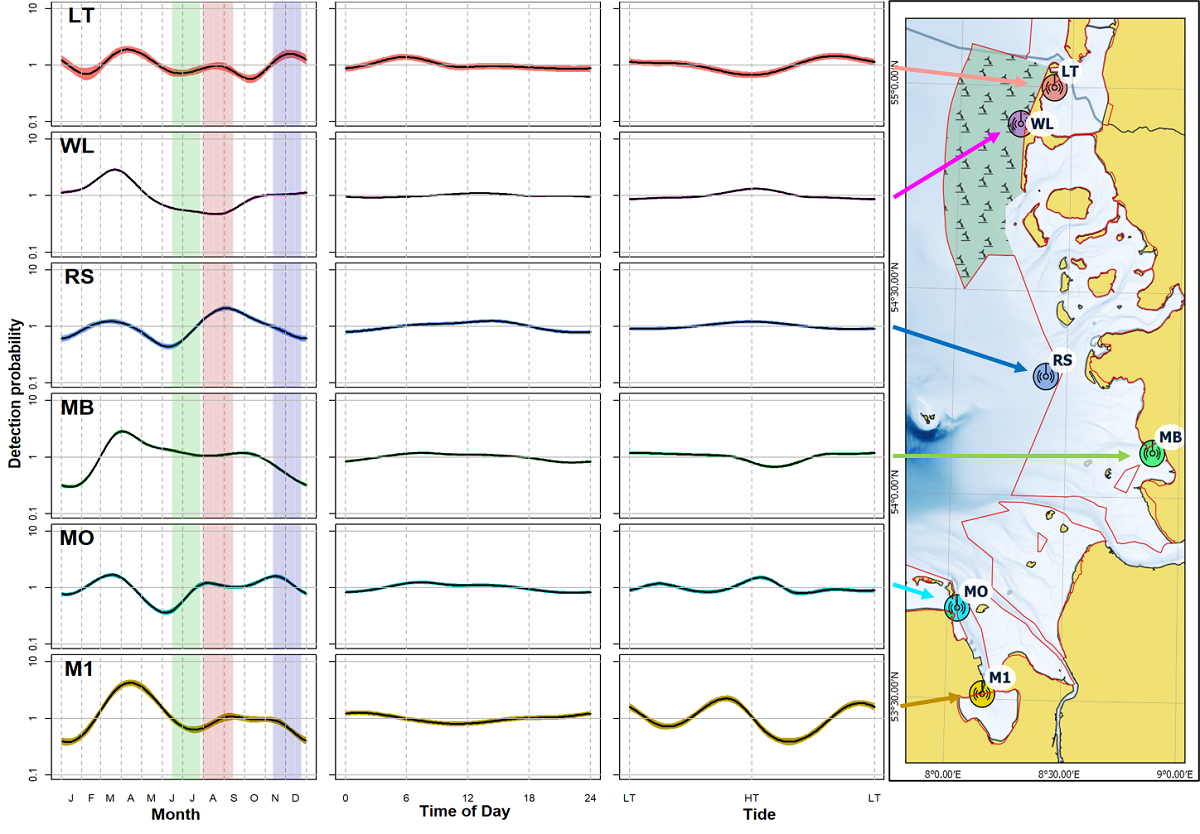 Figure 12. Influence of various environmental variables (month, accounting for season and therefore temperature, daytime for light periods and tide) on the registration of porpoises at the six measuring positions (averaged over the monitoring period). The coloured area around the smooth function displays the area of the 95% confidence interval. The phase of the highest birth rate (June 6 to July 16, according to Hasselmeier et al. (2004) is coloured green, the mating season is red and the time after which the calves start to feed on solid food is blue (adapted from Scheidat et al. (under review)).
Figure 12. Influence of various environmental variables (month, accounting for season and therefore temperature, daytime for light periods and tide) on the registration of porpoises at the six measuring positions (averaged over the monitoring period). The coloured area around the smooth function displays the area of the 95% confidence interval. The phase of the highest birth rate (June 6 to July 16, according to Hasselmeier et al. (2004) is coloured green, the mating season is red and the time after which the calves start to feed on solid food is blue (adapted from Scheidat et al. (under review)).
The acoustic monitoring of the Wadden Sea has generated robust and continuous long-term data sets on harbour porpoise click activity with consistent seasonal patterns for over nine years. The applied models show that harbour porpoises occur year-round in the German Wadden Sea Area and that their presence in specific areas is linked to environmental parameters such as tide. The reasons for this are still not well understood, but as prey availability is thought to be the main driver for porpoise distribution, the inner Wadden Sea waters are likely used to hunt for fish. Analyses of foraging behaviour from PAM data can shed more light on this (Berges et al., 2019; Zein et al., 2019).
This method is especially suitable to assess harbour porpoise occurrence patterns in intertidal waters and an extension of the PAM network would be desirable.
Telemetry (information on individual movement and habitat use)
Aerial surveys, strandings, shore counts and passive acoustic monitoring provide data on the seasonal and inter-annual distribution and density of harbour porpoises in the Wadden Sea and adjacent areas. In general, highest numbers are seen in the northern part in summer, while in the western part of the Wadden Sea this peak is in the winter and early spring (Camphuysen, 2004; Gilles et al., 2009; Scheidat et al., 2012; Gilles et al., 2016; IJsseldijk et al., 2020). This suggests seasonal movements of animals in the area.
One of the most challenging questions is to understand what drives porpoise behaviour, as this is what determines their distribution. The most effective way to understand how porpoises move through their habitat is the tagging of individual animals, with satellite tags or dive recorders (Teilmann et al., 2007; Sveegaard et al., 2011). So far, six harbour porpoise males have been caught in the Wadden Sea and equipped with satellite telemetry tags (Figure 13), which allowed both high resolution tracking over multiple days and low resolution tracking for up to 264 days (van Beest et al., 2018; Stalder et al., 2020). All six animals remained within the Wadden Sea region of Denmark and northern Germany during the whole deployment duration (Stalder et al., 2020). The animals frequently entered tidal channels to the shallow inner parts of the Wadden Sea (Scheidat et al., under review). Only one individual moved further offshore into deeper waters west of Sylt during winter and spring, while the remaining animals displayed high site fidelity (Scheidat et al., under review). Overall, 88% of all locations were in water depths from 1 to 15 m. Furthermore, on average the porpoises spent 67 % (31-98%) of the time observed within the Wadden Sea Area. The high site fidelity and the apparent limited seasonal movements are striking and raise questions about the population structure of harbour porpoises in the Wadden Sea. Could it be that the porpoises in the Wadden Sea have specialized in living in this unique habitat and therefore require a different management framework than the porpoises living further offshore? More tagging studies are needed to conclude on the movements of harbour porpoises in the Wadden Sea and if the preliminary tagging results are representative of the whole region. Such studies will also aid to improve our interpretation of changes in harbour porpoise distribution and population trends on the Wadden Sea but also on North Sea wide scale. In addition, analyses of tissue samples can be used to investigate differences in the utilization of prey resources throughout seasons and beyond national borders.
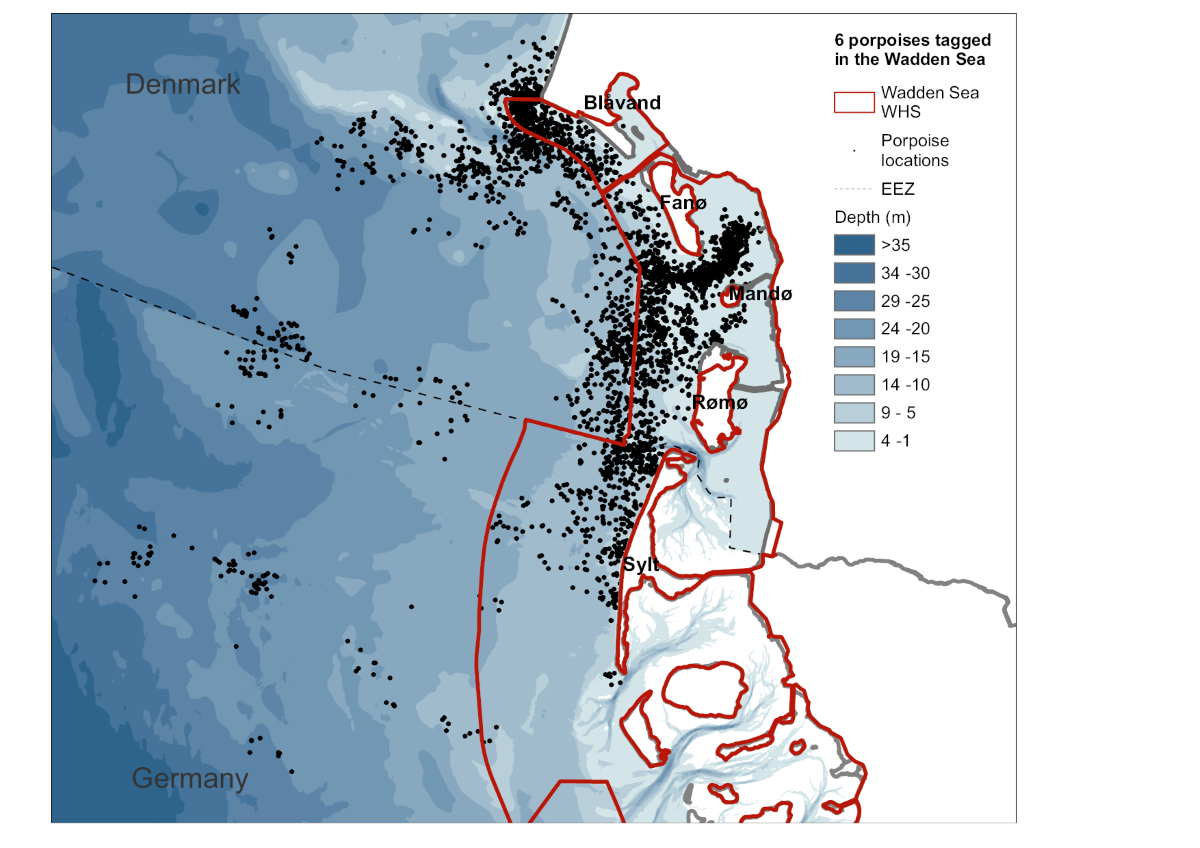 Figure 13. Positions of six tagged harbour porpoises racked in the Wadden Sea Area in 2014 and 2016. The red line shows the borders of the World Heritage Site Wadden Sea.
Figure 13. Positions of six tagged harbour porpoises racked in the Wadden Sea Area in 2014 and 2016. The red line shows the borders of the World Heritage Site Wadden Sea.
Stranding Data / pathological findings in seals and harbour porpoises
General
Harbour seals, grey seals, and harbour porpoises, regularly strand along the Wadden Sea coastline. Occasionally, also other cetaceans (Kinze et al., 2021) and pinnipeds strand, though these species are not indigenous to the Wadden Sea. The collection of stranded marine mammals allows researchers to conduct necropsies which provide valuable information for researchers and conservation managers – on for example population health, environmental contaminant levels, cases of human interaction, and incidence of (zoonotic) disease. Information on stranded marine mammals is collected in national and regional stranding networks in the three Wadden Sea countries, but the number of registered and examined animals and the quality of investigations varies between regions.
Regional stranding data
Denmark
In Denmark, the National Contingency Plan concerning strandings of marine mammals is run jointly by the Danish Environmental Protection Agency, the Danish Nature Agency, the Fisheries and Maritime Museum, the Natural History Museum of Denmark, the Department of Bioscience, Aarhus University, the National Veterinary Institute, DTU and Aalborg University. The stranding network is funded by the Danish government but very much depends on the public reporting stranded marine mammals to the network, which is reflected in the reporting effort.
Germany
In Germany, the stranding network in Schleswig-Holstein has been established after the first seal die-off in 1988/89 (Siebert et al., 2006). All marine mammals have been collected systematically with the stable effort since 1990, funded by the Ministry for the Energy, Agriculture, Environment, Nature Conservation and Digitalisation of Schleswig-Holstein (MELUND) and coordinated by the State Office of Coastal and Nature Protection (LKN.SH) and necropsies are conducted regularly (Siebert et al., 2001; Siebert et al., 2006). Strandings in Lower Saxony and Hamburg are documented by different organisations depending on the species. The existing recording methods are being further developed and are to be combined into a uniform network. If possible, necropsies are being carried out on a selection of stranded seals and harbour porpoises.
The Netherlands
The Dutch stranding network consists of a consortium of organizations and volunteers. The stranding records are open and, for whales, also accessible online (www.walvisstrandingen.nl). Seals are reported to a public database, but a central check for these species is still lacking. An unknown number of stranded seals is discarded without any or only incomplete registration. This process is currently under review and might ameliorate in the near future. Post-mortem examinations on a selection of stranded harbour porpoises are conducted at Utrecht University since 2008, commissioned by the Dutch Ministry of Agriculture, Nature and Food Quality (IJsseldijk et al., 2017), whereas post-mortem examinations of seals are not conducted on a regular basis.
Pathological findings in seals
Denmark
In Denmark, twelve harbour seals were necropsied in the period 2015-2019. None of them showed signs of serious infectious diseases (and PCR was negative for morbillivirus and influenzavirus). More than two-thirds of the seals showed varying respiratory lesions with parasites (lungworm and heartworm) as the primary pathogen (Figure 14) (Thøstesen et al., 2016; Thøstesen et al., 2017; Thøstesen et al., 2018; Thøstesen et al., in prep.). In the same period three grey seals stranded in the Danish Wadden Sea Area, but none of them was necropsied (Thøstesen et al., 2016; Thøstesen et al., 2017; Thøstesen et al., 2018; Thøstesen et al., in prep.)
 Figure 14. Harbour seal (Phoca vitulina) with respiratory lesions presumably due to infection with parasites. (Photo: TTF, Fiskeri- og Søfartsmuseet. Esbjerg)
Figure 14. Harbour seal (Phoca vitulina) with respiratory lesions presumably due to infection with parasites. (Photo: TTF, Fiskeri- og Søfartsmuseet. Esbjerg)
Germany
Federal State of Schleswig-Holstein
In Schleswig-Holstein, 50-80 harbour and all grey seals are necropsied each year. For harbour seals, findings in younger animals were similar to those in Lower Saxony. In older animals, displacement of the intestine was frequently found (Ludes-Wehrmeister et al., 2020). Also, fatal sexual encounters with male grey seals have been found to lead to the death of female harbour seals in Schleswig-Holstein (Rohner et al., 2020).
Grey seal predation and cannibalism has now been increasingly recognized and need to be further monitored (Leopold et al., 2015b; van Neer et al., 2021). Parasitosis of the digestive tract was documented in grey seals (Lakemeyer et al., 2020).
Federal State of Lower Saxony
In Lower Saxony, 28 harbour seals were examined in 2020. Almost all of them were juveniles that were either found dead or had to be euthanized.
In addition to pulmonary and intestinal parasitoses, viral intestinal infections, bacterial pneumonia and umbilical infections were detected. Inflammations in the mouth area were found remarkably frequently. One animal showed high-degree changes in the brain, which were probably due to a vitamin B1 deficiency. Two animals had suffered blunt trauma.
All animals tested negative for influenza virus and morbillivirus.
The Netherlands
There is no structural post-mortem project on seals in the Netherlands, although 15 seals from the Wadden Sea Area were necropsied in 2019 following the MSC Zoe plastic spill. Most of these seals had pulmonary lesions and two died following blunt-forced trauma with unknown origin (IJsseldijk et al., 2020; Schop et al., 2020).
Pathological findings in harbour porpoises
General
Most harbour porpoises stranded in the Wadden Sea Area are likely originating from the North Sea population. Stranding records of harbour porpoises (n=16,181) from around the North Sea over a 28-year period (1990-2017) were analyzed to determine spatiotemporal mortality and demographic trends (IJsseldijk et al., 2020). The annual number of stranding incidences has increased since 1990, with a notable steeper rise, particularly in the southern North Sea, since 2005. A high density of neonatal strandings occurred especially in the eastern North Sea, indicative of areas important for calving. Large numbers of juvenile males stranded in the southern parts indicate a population sink or reflect higher male dispersion.
Denmark
For Denmark, in the period 2015-2019, a total of 38 harbour porpoises were reported from the Danish Wadden Sea (Figure 15). Nine of these porpoises were necropsied, two of which showed signs consistent with drowning (bycatch). In two-thirds of the animals, various parasites were identified, mainly in the respiratory tract. One of the porpoises was newborn or stillborn (Thøstesen et al., 2016; Thøstesen et al., 2017; Thøstesen et al., 2018; Thøstesen et al., in prep.).
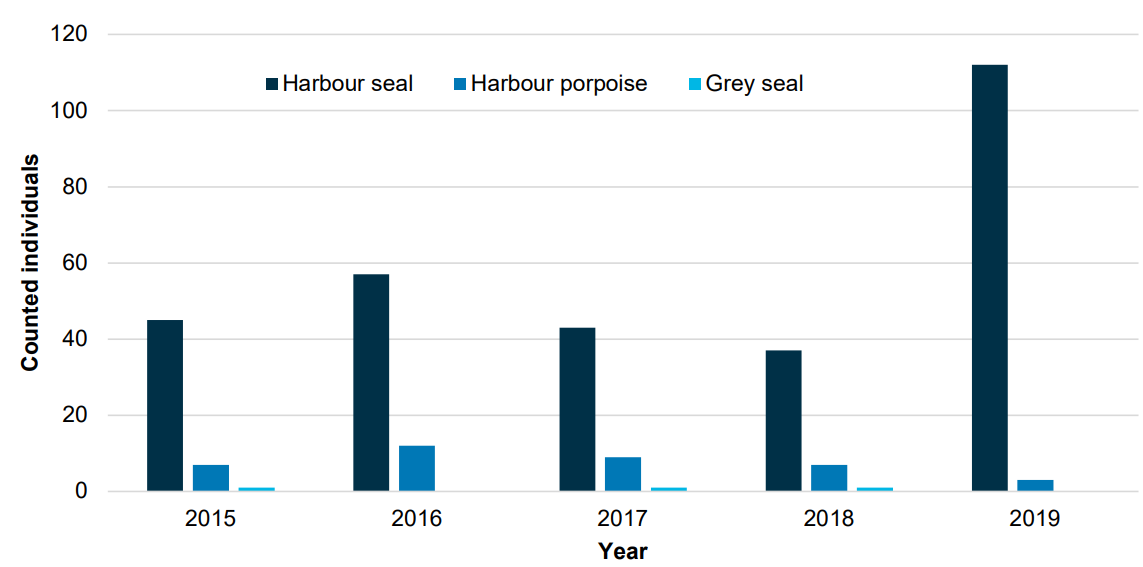 Figure 15. The number of stranded harbour seals (Phoca vitulina), Harbour porpoises (Phocoena phocoena) and grey seals (Halichoerus grypus) reported from the Danish Wadden Sea Area to the Danish National Contingency Plan concerning strandings of marine mammals in the period 2015 to 2019.
Figure 15. The number of stranded harbour seals (Phoca vitulina), Harbour porpoises (Phocoena phocoena) and grey seals (Halichoerus grypus) reported from the Danish Wadden Sea Area to the Danish National Contingency Plan concerning strandings of marine mammals in the period 2015 to 2019.
Germany
Federal State of Schleswig-Holstein
Between 1990 and 2020 a total of 2,905 harbour porpoises collected on the coast of Schleswig-Holstein were examined. The most common cause of death was bronchopneumonia, septicemia and neonatal death (Siebert et al., 2001; Siebert et al., 2012). Several potentially pathogenic and zoonotic bacteria were isolated including Brucella sp., Escherichia coli, beta-haemolytic streptococci and Staphylococcus aureus (Siebert et al., 2009).
The Netherlands
Necropsies on more than 600 freshly stranded harbour porpoises between 2008 and 2019 in The Netherlands, revealed that infectious diseases were by far the most common cause of death, mainly for adults. The most frequent anthropogenic cause was bycatch, mainly juveniles being affected and peak periods in March and September. Other anthropogenic causes of mortality, like ship strikes and marine debris entanglement, were infrequently found.
Specialities in the Wadden Sea Region
In November 2019 four subadult long-finned pilot whales (Globicephala melas, three males and one female) were found dead in the Wadden Sea of Lower Saxony (Figure 16). Necropsies revealed that the whales were in good nutritional condition, however, the gastrointestinal tract was completely empty. Food or foreign body components were not found. It can be assumed that the animals strayed into the North Sea, subsequently stranded and died of a resulting cardiovascular collapse. No indication of specific infectious agents was found. With the help of residue analysis, very high concentrations of non-dioxin like polychlorinated biphenyls (ndl-PCBs), palars and dichlorophenylthrichlorethane (DDT) could be detected. High levels of mercury (Hg) and cadmium (Cd) were detected in the liver and kidney tissue of the animals during elemental analysis. From a differential diagnostic point of view, a health impairment of the animals due to the high pollutant loads cannot be ruled out as not all necessary investigations could be conducted. In addition, investigations showed indications of Pestivirus in all four pilot whales.
 Figure 16. One of the long-finned pilot whales (Globicephala melas) stranded in the Wadden Sea of Lower Saxony, Germany; (Photo: LAVES)
Figure 16. One of the long-finned pilot whales (Globicephala melas) stranded in the Wadden Sea of Lower Saxony, Germany; (Photo: LAVES)
A Cuvier's beaked whale (Ziphius cavirostris, an adult male, length 5.82 meters, weight 2.5 tons, good nutritional status) stranded in Denmark on Lakolk Beach (Rømø, Denmark) in February 2020. The necropsy showed that the whale may have died of purulent pneumonia, leading to agonal septicaemia. In addition, various parasites were found including nematodes (Crassicauda crassicauda) in the kidneys, as well as various parasites in the intestines and abscesses in the blubber. In the anterior ventricle there were two small pieces of plastic, to which, however, death cannot be attributed (Alstrup et al., 2021).
Threats to marine mammals in the Wadden Sea
Anthropogenic induced health impacts on marine mammals
Marine mammals are exposed to an increasing number of anthropogenic influences within the inner waters of the Wadden Sea, as well as the offshore parts. These influences include bycatch, shipping, offshore-wind farm construction, ammunition (inherited waste), recreational and military activities, fisheries and aquaculture, chemical pollution, and marine litter (Siebert et al., 2012).
A study on bycaught and stranded harbour porpoises from the southern North Sea revealed that the criteria previously used to identify bycaught animals, namely ‘a favourable health status’, ‘the absence of disease’, and ‘good nutritional conditions’, did not apply to the majority of bycaught porpoises, pointing to overall poor health of harbour porpoises in the southern North Sea (IJsseldijk et al., 2021). Assuming that stranded animals with various diseases are not being identified as bycatch will therefore inevitably result in underestimated bycatch numbers (Siebert et al., 2020; IJsseldijk et al., 2021). Investigations of female harbour porpoises collected on the North Sea coast of Schleswig-Holstein showed that the mean age at death of female harbour porpoises in the North Sea is only 5.7 (± 0.27) years, although harbour porpoises can live up to 25 years and reach sexual maturity with 4.95 (±0.6) years (Kesselring et al., 2017). It is still unclear why some porpoises die so early, but it can be expected that this will have a negative effect on the harbour porpoise population development in the North Sea, including the Wadden Sea (Kesselring et al., 2017).
As it is shown above single effects are partly being investigated, increased research effort is needed to also investigate the cumulative effects of those activities on all three marine mammal species. The impact of the numerous threats can vary from direct death due to bycatch, acoustic or blunt trauma to indirect or chronic changes as affecting the immune or endocrine system, hearing and reproductive impairment, and increase of infectious diseases and starvation, which may ultimately reduce the lifespan of an individual. To take appropriate protection and management measures and to assess the capacity of the Wadden Sea populations to adapt to anthropogenic activities, including climate change, long-term and combined, transnational health assessments of Wadden Sea marine mammals are needed.
Noise Pollution
Harbour porpoises are not only affected by environmental factors, but also by anthropogenic activities causing underwater noise (Lucke et al., 2009; Dähne et al., 2013; Wisniewska et al., 2018). Similarly, there is growing evidence that underwater noise has adverse effects on seals (Russell et al., 2016; Aarts et al., 2018; Mikkelsen et al., 2019).
All marine mammal species inhabiting the Wadden Sea are potentially affected by continuous noise from ship traffic. Seals were exposed to ship noise up to 20.5% of their time at sea (Mikkelsen et al., 2019) and harbour porpoises between 17 and 89% (Wisniewska et al., 2018) of their time at sea, causing behavioural changes, such as reduced foraging. The consequences on an individual and population level are not yet evaluated but matter of current research.
Impulsive noise (e.g., from the construction of offshore wind turbines, military sonar or seismic surveys) can also affect harbour porpoises and seals, causing behavioural changes (disturbance) or hearing threshold shifts (Kastelein et al., 2016; Sills et al., 2020). Especially for harbour porpoises, which depend on echolocation as the main sensory system, reduced hearing can have major effects, such as reduced foraging efficiency. An example of the impact of impulsive noise comes from a study monitoring construction work in Eemshaven leading to reduced harbour porpoise vocalisations, and seals showing avoidance behaviour (Brasseur et al., 2011)
PAM was used to assess the impact of wind farm construction activities on harbour porpoises and showed a decrease in harbour porpoise detections during pile driving (Carstensen et al., 2006; Tougaard et al., 2009; Scheidat et al., 2011; Dähne et al., 2013; Brandt et al., 2018). Telemetry studies on seals also showed behavioural changes during pile driving (Brasseur et al., 2011; Whyte et al., 2020).
Baltzer et al. (2020) investigated the effect of innovative construction technology (anchor vibration) during the construction of seed mussel collectors in the Wadden Sea. Underwater noise was recorded with increasing distance to the construction site to estimate sound propagation and thus the disturbance potential to the marine fauna. Behavioural thresholds for indigenous species of marine mammals in the Wadden Sea as well as representative fish species were used to determine the effect radii of vibration noise. Vibration noise can be defined as continuous sound lasting for up to 55 s with most energy below 1 kHz. The study showed that the detected vibration noise might exert a behavioural reaction on a local scale. Marine mammals could be affected by the construction operations up to a distance of 375 m and fish up to a distance of 766 m.
The zones of responsiveness for vibration operations are relatively small, compared to pile driving, providing a good alternative method. The study also highlights the importance of acoustically monitoring the coastal environment as it allows assessment of potential disturbance effects from introduced noise from anthropogenic activities and whether anthropogenic use complies with guidelines.
Fisheries
The fishery has both direct and indirect impacts on marine mammals such as bycatch, habitat and prey loss. Today, the most targeted species in fisheries in the Wadden Sea are shrimp, shellfish and on a smaller scale demersal fish species like sandeel and flatfish (Baer et al., 2017). In contrast to beam trawls mainly used in flatfish fisheries, the shrimp beam trawl is comparatively lighter, commonly used without chase chains and thus less destructive for the seabed (Schulte et al., 2015). Nevertheless, bottom trawling fishing methods impact seabed habitats and sensitive – esp. epibenthic – species (Schulte et al., 2015). These impacts should be minimised to maintain a stable habitat and food web.
Industrial fishery (i.e. fishing on species used for the production of fishmeal and fish oil) on sandeel (Ammodytes spp.) takes place in the offshore area of the Wadden Sea (Sell et al., 2011). Sandeels are shoaling fish, rich in lipid (MacDonald et al., 2019) and a crucial prey in the North Sea for different predator species such as harbour porpoises, harbour seals and grey seals (Santos et al., 2004; MacLeod et al., 2007; Sharples et al., 2009; Hammond & Wilson, 2016). Based on a limited opportunity of energy storage, e.g. harbour porpoises rely on a high prey abundance and/or prey quality since they have a high foraging and metabolic rate (Ransijn et al., 2019). Herr et al. (2009) recommend exclusion areas for industrial fisheries to reduce foraging competition between harbour porpoises and industrial fisheries.
Accidental entanglement in fishing gear is considered a serious threat to the harbour porpoise population and also a threat to seals in the North Sea (ICES, 2020). Currently, experts from an OSPAR working group update thresholds for bycatch rates for harbour porpoise and grey seals for the North-east Atlantic Area (OSPAR, 2021). One approach to reducing the bycatch of marine mammals is to attach deterrent devices such as pingers to the fishing gear, but reliable data on the effectiveness are still lacking (WGBYC, 2020). Moreover, those devices are associated with different issues, which are described in detail by ICES (ICES, 2020). Thus, Ridoux (2020) recommends this approach only in combination with fishery closures and alternative fishing gear. In accordance, it is highly recommended to ban set net fishery at least inside protected zones like, e.g. the FFH-areas (Jensen et al., 2017; see 2017 QSR thematic report on marine mammals), as it is already partly applied in the whale sanctuary in Schleswig-Holstein (ASCOBANS, 2018).
Furthermore, it is essential to establish a reliable, processed and easily accessible database and maps for the Wadden Sea, to notice changes in fishing intensity (e.g. due to displacement from offshore wind park areas) promptly. In addition, it is essential to improve the process to implement fishery management measures in a timely manner (see BfN (2021)).
Marine litter
Between 2018 and April 2021, three carcasses of marine mammals were found at the German coastline of Schleswig-Holstein (SH) which had ingested anthropogenic litter: one sharp-edged fragment of a black plastic object in the stomach of a grey seal (Figure 17 A), one piece of a sweet wrapper in the stomach of a harbour porpoise (Figure 17 B), and some fibres of dolly rope were present in the colon of another grey seal (Figure 17 C) (ITAW, unpublished data). Furthermore, in three cases (one harbour porpoise, one harbour seal and one grey seal) blue dolly ropes (fibres of abrasion protection used in fisheries) were detected. However, those fibres were found in open wounds or on macerated carcasses, thus post-mortem exposure could not be excluded.
Furthermore, two small plastic objects were found in the first stomach compartment of a stranded Cuvier’s beaked whale at Rømø in 2020. One-piece was flat and 5 x 8 cm in size, while the other was a ribbon-like object 2 x 35 cm in size (Alstrup et al., 2021).
The evaluation of 654 stomachs of harbour porpoises collected at the Dutch coastline between 2003 and 2013 revealed man-made litter objects in 47 cases (van Franeker et al., 2018). In those 7% out of the investigated stomachs, 76 objects were determined: 71 plastic items and three pieces of paper, one fishing hook and one non-synthetic rope (van Franeker et al., 2018). Moreover, 40 stomachs of harbour porpoises stranded at the Dutch coastline in 2019 were analysed, since consequences after the average of the MSC Zoe on the 2nd January 2019 were anticipated. In 12.5% out of these examined stomachs, artificial objects were detected, albeit no correlation between the number of objects and mortality was determined (Bravo Rebolledo, 2021). Both aforementioned studies focussed on items larger than 1 mm, but the visual assessment was conducted beforehand.
Analysing nine faecal samples of seals collected on the Lorenzenplate (Germany) in 2019 revealed a microplastic burden in every sample (1 – 8 particles per sample) (Philipp et al., 2021). Nine randomly investigated intestinal samples of harbour and grey seals found at the German coastline of SH between 2013 and 2019 showed that all samples were burdened. In total, 177 microplastic particles (58 fibres, 119 fragments) were found in those nine samples (Philipp et al., 2021). Another approach focussing on microplastic occurrence (>100 µm) in 14 intestinal samples of harbour porpoises stranded in the German North Sea between 2014 and 2018 revealed a burden of microplastics in twelve individuals (Philipp et al., 2021).
According to these newer findings and the listed cases of marine litter exposure to different biota species, incl. marine mammals, in the Wadden Sea QSR from 2017, there is still the need to further report and record findings of ingested or entangled macrolitter. In addition, it is advisable to focus on microplastics in the future due to the uncertain health effects of microplastics and nanoplastics on marine mammals. Furthermore, those top predators are ideal candidates to monitor the microplastic pollution in waters inhabited by them and in their prey species.
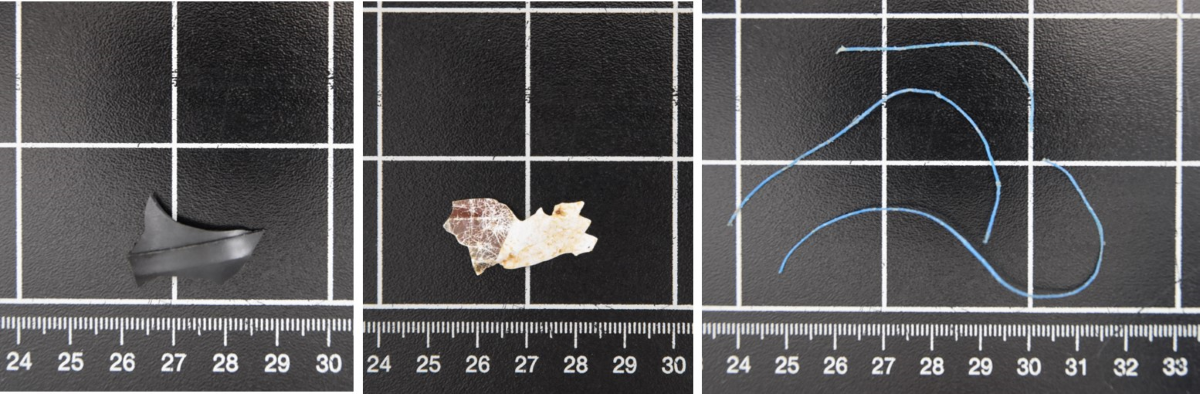 Figure 17. Found anthropogenic litter items in marine mammals. A) Sharp-edged plastic piece, B) Piece of sweet wrapper and C) fibres of dolly ropes. (Photo: Philipp et al., 2021).
Figure 17. Found anthropogenic litter items in marine mammals. A) Sharp-edged plastic piece, B) Piece of sweet wrapper and C) fibres of dolly ropes. (Photo: Philipp et al., 2021).
Climate change
Climate-related changes in the Wadden Sea Area are likely to impact grey seal, harbour seal and harbour porpoise. Rising sea levels, changing weather patterns, and reduced sediment supply to the tidal basins will alter the physical habitat of the Wadden Sea (Woth et al., 2006; Reise & van Beusekom, 2008; Kabat et al., 2012; Lodder et al., 2019). Suitable haul-out sites for resting and breeding seals can become rarer and more frequent storm surges can increase the risk of drowning for grey seal pups (Brasseur et al., 2014a).
One of the most relevant impacts is the likely change in prey availability. Changes in ocean circulation, sea surface temperature, CO2 levels, salinity and increased acidification, can alter fish phenology, biomass and community (Tulp et al., 2008; van der Veer et al., 2015; Meyer et al., 2016; Van Walraven et al., 2017). Porpoise habitat use in the Wadden Sea is likely linked to tides and the migratory movements of anadromous fish (Wenger & Koschinski, 2012; Weel et al., 2018; Zein et al., 2019). Both seals and porpoise depend on the North Sea for foraging and will be impacted by large scale ecosystem changes. In addition, the appearance of new species, including other top predators, could lead to competition over prey or other resources (Learmonth et al., 2006).
Temperature increase can cause thermal stress but to date, there is little evidence for this in marine mammals of the Wadden Sea. Animals will likely try to stay in their preferred thermal habitats and adapt their distribution if needed (MacLeod, 2009; Simmonds & Eliott, 2009; Lambert et al., 2011). Seals have shown a shift in their breeding phenology with an earlier start of their pupping season between 0.59 and 0.88 days (harbour seal) and 1.3 days (grey seal) each year (Reijnders et al., 2010a; Osinga et al., 2012; Brasseur et al., 2014b). It is thought that this is an adaptation to changes in their habitat, but it is unclear if this is linked to larger-scale climatic changes.
Climate change is also associated with increases in diseases, new parasites and epizootics through the introduction of new pathogens (Poulin & Mouritsen, 2006; Van Bressem et al., 2009; Goedknegt et al., 2015; Schade et al., 2016; Sonne et al., 2020). This is particularly problematic if animals are already compromised by high levels of pollutants (Jepson et al. 2005, Verborgh et al. 2019).
The impact of climate change on marine mammals is difficult to predict as there are many potentially confounding variables. Marine mammal populations can adapt to habitat change by natural selection or phenotypic plasticity, changes in distribution and population size (Fontaine et al., 2010). Shifts in distribution can also be due to normal variability in habitat use (Fietz et al., 2016) or related to other existing anthropogenic pressures. Identifying the key drivers requires a better understanding of the relationship between marine mammals and climatic indices in the Wadden Sea (Simmonds & Eliott, 2009; Nunny & Simmonds, 2019).
Marine spatial planning of offshore wind farms and the potential for habitat fragmentation and loss
Both seal species resident to the Wadden Sea, are dependent on the North Sea for foraging and the increased anthropogenic use of their habitats could negatively affect them. Political decision-makers have set new goals aiming at the transformation of the energy sector towards renewable energies. Offshore wind farms play a crucial role in achieving these goals, leading to a demand for suitable areas.
While the Wadden Sea is a key habitat for seals, especially for resting, pupping and moulting, the role of offshore habitats for migration or foraging is less well understood. Tracking studies in the past decades of both harbour and grey seals from the Wadden Sea, have however clearly demonstrated their intensive use of the North Sea (Tougaard et al., 2008; Brasseur, 2017; Aarts et al., 2019; Peschko et al., 2020), demonstrating a large potential of overlap between the habitat utilised by the seals and the areas considered for the construction of offshore wind farms or other human use (e.g. Figure 18).
Information on potential effects of offshore human activities is lacking. In particular information on a large spatial scale is needed which includes data on habitat fragmentation, barrier effects and the complete loss of habitat due to potential avoidance by seals. Impacts could have different causes, including the physical presence, increased acoustic pollution, as well as ship traffic (Brasseur et al., 2009; Brasseur et al., 2012; Schuster et al., 2015; Mikkelsen et al., 2019). Future studies should also consider indirect and positive effects including for example the suggested changes in food availability due to the reef effect or the prohibited fishing within offshore wind farms or potential large scale meteorological changes due to the removal of kinetic energy by the wind farms (e.g. Boon et al. (2018).
A recent study on harbour seals using high-resolution multi-sensor bio-logging tags showed that feeding occurs not only at the offshore locations but also during travel from and to the haul out in the Wadden Sea (Vance et al., 2021). Studies combining information from all regions bordering the Wadden Sea would potentially include the variation in habitats and help to elucidate which effects the future development of offshore wind farms in the vicinity of the Wadden Sea ecosystem will have on the local seal stocks.
The extension of offshore wind farms in the North Sea may also adversely affect harbour porpoises. When the wind turbines are driven into the seabed, harbour porpoises avoid the construction sites up to a distance of ca. 20 km (Dähne et al., 2013; Brandt et al., 2018). It is still unclear if harbour porpoises return to the area following the construction of the offshore wind farm to the same extent as before. There are contradicting studies that either report an increase or decline of echolocation activity around the offshore wind farms (Carstensen et al., 2006; Scheidat et al., 2011). Depending on the construction schedule and the length of breaks between pile-driving events, the construction of wind farms can have consequences on the population level, in particular when they are built-in important foraging grounds (Nabe‐Nielsen et al., 2018).
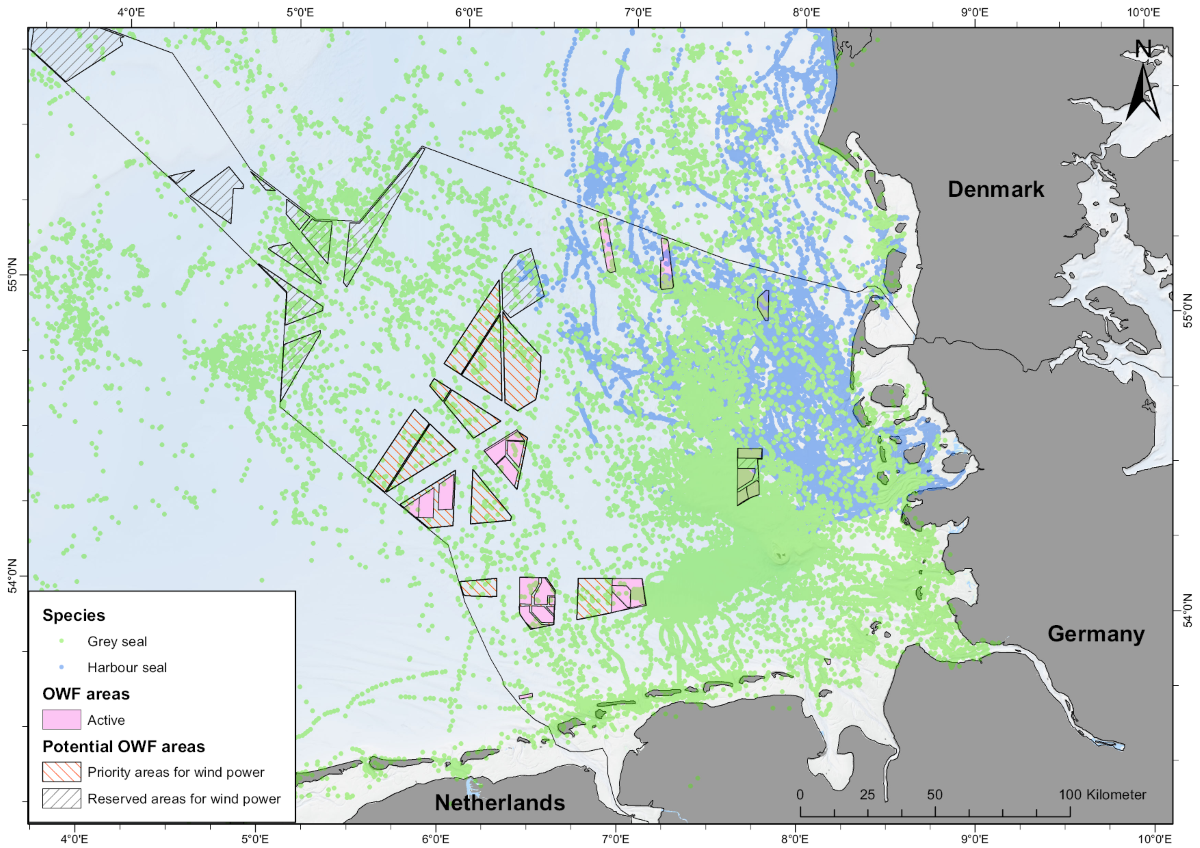 Figure 18. Position data from seals tagged in the Wadden Sea of Schleswig-Holstein and on Helgoland colour coded by species (ITAW, unpublished data), showing the large potential overlap between the habitat utilised by the seals and the areas considered for the construction of offshore wind farms. Rectangles represent areas with active or planned offshore wind farms as well as priority areas considered for future developments (Photo: BSH, 2021).
Figure 18. Position data from seals tagged in the Wadden Sea of Schleswig-Holstein and on Helgoland colour coded by species (ITAW, unpublished data), showing the large potential overlap between the habitat utilised by the seals and the areas considered for the construction of offshore wind farms. Rectangles represent areas with active or planned offshore wind farms as well as priority areas considered for future developments (Photo: BSH, 2021).
3. Assessment
General
The harbour seal (Phoca vitulina), grey seal (Halichoerus grypus) and harbour porpoise (Phocoena phocoena) are regarded as indigenous marine mammals in the Wadden Sea. All three species are listed in Annex II of the EU Habitats Directive and special areas have been designated for their conservation. Harbour porpoises are also listed under Annex IV, which requires strict protection throughout the entire range of the species in European waters. Moreover, harbour seals and grey seals are listed in Annex V, thus their exploitation should be regulated to be compatible with maintaining or attaining a good environmental status. In addition, harbour seals are protected through the Agreement on the Conservation of Seals in the Wadden Sea, or Wadden Sea Seal Agreement (WSSA), concluded under the Bonn Convention on the Conservation of Migratory Species of Wild Animals. Since the signing of the Wadden Sea Seal Agreement, grey seals have become more abundant and have been included in the Seal Management Plan elaborated under the Wadden Sea Seal Agreement (though they are not covered by the Agreement itself). The harbour porpoise is protected according to the Agreement on the Conservation of Small Cetaceans of the Baltic, North East Atlantic, Irish and North Seas (ASCOBANS).
According to the Wadden Sea Plan (Common Wadden Sea Secretariat, 2010) the targets for each marine mammal species, which are consistent with the national Conservation Objectives under the Habitats Directive as well as World Natural Heritage criterion X, are as follows:
-
Viable stocks and a natural reproduction capacity of the harbour seal, including juvenile survival;
-
Viable stocks and a natural reproduction capacity of the grey seal, including juvenile survival;
-
Viable stocks and a natural reproduction capacity of the harbour porpoise; Conservation of habitat quality for the conservation of the species.
To allow an assessment of these targets, Reijnders et al. (2009) state that a population is considered viable if it “maintains its vigour and its potential for evolutionary adaptations”. To do this a population has to be large enough to withstand catastrophic events, such as a mass mortality event, and to have enough genetic variability that its evolutionary potential is not hindered. The use of term “stock” is used in fishery management to describe a living resource from which catches are taken. The term “usually implies that the particular population is more or less isolated reproductively from other stocks of the same species and hence self-sustaining” (FAO, 1997).
Assessment for harbour seals
The Wadden Sea harbour seal population has been recovering from severe overhunting in the 20th century. The estimated population size grew from less than 4,000 in 1975, when the trilateral coordinated monitoring started, to 40,000 animals in 2012, and as such it is currently the largest harbour seal population in Europe. Since ca. 2012, the population growth has stagnated, despite a growing pup production (~10,000 in 2020), representing more than 25% relative to the estimated population size. Due to the sudden change and a lack of reduction in pup production, it is unlikely that the lack of population growth is a result of the population approaching carrying capacity, where food would be the limiting factor. If access to sufficient energy was the limiting factor, we would expect a lowering in pup production, poor body condition and an increasing number of stranded seals, which has not been confirmed. Currently, it is uncertain which mechanisms are involved. In theory, the seals may have, rather suddenly, changed haul-out patterns during moult or there is unrecorded mortality in the population.
The following point may be part of explaining this unexpected population development:
-
There seems to be regular exchange with the harbour seal colonies in the Dutch Delta area where numbers have grown since the late 20th century from 20 animals to over 1,200 seals observed during the moult in 2019. This growth is remarkable as pup counts are too low to explain this recovery. The likely link between the Wadden Sea and British Isle populations should be further analysed.
-
Though not recorded in the population recently, an immediate threat to the population would be the recurrence of a PDV epizootic, which killed ca. 50 % of the population in 1988 and 2002.
-
Pollution is presently not regarded as a major issue for the survival and reproduction of the population but has not been studied recently.
-
Due to a lack of detailed monitoring, bycatch cannot be quantified.
-
The effects of growing noise pollution caused by anthropogenic activities, for example, due to increasing shipping activities and offshore wind farms in the North Sea where the animals from the Wadden Sea are feeding, are unclear. Further increase in activities is expected in the near future, during the transition to renewable energy.
-
Within the Wadden Sea guidelines for the increasing recreational activities, including organized seal safaris, should be drafted and regulations should be implemented to reduce disturbance of breeding, moulting and resting seals. Further adherence to regulations should be closely monitored. Protection of the seals in sensitive periods can potentially ensure a higher resilience of the population to affront future changes. Following new regulations, rehabilitation of seals in the Dutch Wadden Sea has been reduced as this was at a level causing concern, but would not explain the change in population growth.
-
Grey seals have been reported to predate on harbour seals in the region, but this has not been quantified.
To understand the current development in the Wadden Sea harbour seal population, an integrated study on what factors affect population growth is needed.
Assessment for grey seals
Clear growth in the number of grey seals has been observed in the Wadden Sea Area (including Helgoland), since immigrants from the UK started to recolonize the area in the mid-20th century. Numbers counted during the moult have reached a total of over 9,000 and almost 2,000 pups were counted in the season of 2020-2021, which is the highest numbers counted so far. Grey seals have been observed throughout the Wadden Sea countries, though approximately 75% of the moulting seals and 58% of the pups are observed in the Dutch Wadden Sea. As a regular exchange occurs between the Wadden Sea and the Dutch Delta and the UK grey seal population, the grey seals in the Wadden Sea Area are considered as part of a North Sea population.
The grey seals in the Wadden Sea breed on suboptimal sites from which the pups can be flushed off during a winter storm, causing mortality. This is mainly due to the lack of sufficiently high sandbanks throughout the tidal mudflats of the Wadden Sea in combination with the still increasing rate of suitable habitat deemed lost due to anthropogenic utilisation. One major factor limiting the available habitat is tourism occupying vast areas like the beaches of the Wadden Sea islands, which would otherwise be a safe place to raise pups. Besides this, the increase in shipping, as well as water-based recreational activities such as kite surfing likely, amplifies this effect.
Grey seals are much less susceptible to the PDV epizootic that previously killed 50% of the harbour seals. Other risks, including pollution, bycatch and disturbance, are similar to the risks observed in harbour seals. New regulations for rehabilitation also apply to this species of seals in the Dutch Wadden Sea as this was at a level causing concern.
Assessment for harbour porpoises
The SCANS surveys in 1994, 2005, and 2016 provide abundance estimates for the larger North Sea population. These surveys showed no indications of a change in abundance of the North Sea population over the last three decades, but large-scale changes in distribution have led to an increase of harbour porpoises in the southern North Sea over the last decades. Currently, it is not possible to assess if there is a viable stock of harbour porpoises in the World Heritage Site, as site-specific information on abundance and reproductive capacity is lacking.
A recent trend analysis based on aerial survey data from the German national monitoring scheme revealed a decrease in harbour porpoise abundance in northern areas of the Wadden Sea region west of Sylt and an increase in more southern areas between Borkum and the Elbe estuary (Nachtsheim et al., 2021).
Six harbour porpoises have been tracked by satellite tags covering all months of the year within the Wadden Sea region of Denmark and northern Germany. The animals frequently entered the shallow inner parts of the intertidal Wadden Sea Area and spent the majority of their time in the Wadden Sea Area. This high site fidelity and the apparent limited seasonal movements are striking and raise questions about a potential separate population structure of harbour porpoises in the Wadden Sea.
Tracking data and passive acoustic monitoring confirm that porpoises use the Wadden Sea Area, including the intertidal waters. Porpoise distribution is thought to be primarily driven by prey availability, as one can assume that they are successfully foraging in this complex habitat. While passive acoustic monitoring data indicate that porpoise occurrence is linked to tidal patterns, our understanding of the factors driving their habitat use is still poor. This also makes it difficult at this point to assess the habitat quality of the site.
The North Sea harbour porpoise population is subject to a large number of potentially harmful anthropogenic activities. These include bycatch in gillnets and pollution, as well as growing noise pollution caused by increased shipping activities and the construction of offshore wind farms. Further increase in anthropogenic activities is expected in the near future, during the transition to renewable energy. The potential impact of these and other threats on the harbour porpoise in the Wadden Sea Area can currently not be assessed.
To assess if harbour porpoises in the Wadden Sea Area are a “viable stock with a natural reproductive capacity”, baseline data on local population structure, health, habitat use, abundance and distribution is needed. To assess to what degree the “habitat quality” is sufficient to conserve the species, it is necessary to understand the link between prey availability in the Wadden Sea and harbour porpoises. This is particularly true if the Wadden Sea supports a local population.
A coordinated trilateral monitoring scheme is the best way forward to ensure that monitoring efforts are effective and will provide the necessary data for the long-term assessment of the harbour porpoise in the Wadden Sea Area.
4. Recommendations
Recommendations for monitoring and research (based on the Seal Management Plan)
In Denmark, Germany, and the Netherlands, coordinated monitoring of the harbour seals (Phoca vitulina) and grey seals (Halichoerus grypus) in the Wadden Sea has been carried out since 1975 and 2008, respectively. This provides a long-term record of population trends in terms of population size and distribution of these species. By signing the Agreement on the Conservation of Seals in the Wadden Sea, the three countries emphasized the importance of a coordinated monitoring programme for the harbour seal. On this basis, the Seal Management Plan (SMP) defines a minimum effort of five aerial surveys each year for each species – three during pupping season and two surveys during the moulting period. After their recolonizations of the Wadden Sea, the SMP was extended to include the grey seals with similar numbers of aerial surveys. But due to different timing in pupping and moulting for grey seals they are monitored in five separate aerial surveys accordingly.
It is recommended by the Seal Management Plan to conduct extra flights every five years. This recommendation is based on the results of several studies demonstrating that changes in breeding phenology of harbour and grey seals in the Wadden Sea have taken place over the past decades (Reijnders et al., 2010a; Cordes & Thompson, 2013; Brasseur et al., 2015; Brasseur, 2017). The mean birth date of harbour seals has shifted and the birth peak occurred 25 days earlier in 2009 than in the 1970s. Similar shifts were observed in grey seals between 1985 and 2013. Importantly, the shift was continual but not constant over the study period (Reijnders et al., 2010a; Brasseur, 2017). Hence, the timing of pupping in seals is a variable trait under the likely influence of factors such as maternal nutritional status, population demography and density or possibly global warming (Abt, 2010). Accordingly, the seal monitoring programme should be able to accommodate inter-annual variation in the timing of pupping and detect sustained trends in this timing. It is reasonable to expect that the observed changes in breeding phenology are accompanied by changes in the timing of the moult. On this basis, it remains recommended that three extra coordinated survey flights are conducted every fifth year: two extra flights during the pupping season to track changes in breeding phenology and one extra survey flight during the moulting season to detect changes in the timing of moult.
Though these extra flights were regularly carried out before 2000, this recommendation has not been followed since and it is therefore not clear how well the surveys for both grey and harbour seals are timed in accordance to moulting and pupping phenology. Therefore, extra flights or other studies that can determine changes in timing are needed in order to maintain a solid and scientific sound monitoring scheme.
As different trends in the number of harbour seal adults in comparison to pups counted in the Wadden Sea have been recorded (see assessment), it is crucial to further assess this topic. Besides additional aerial surveys, further research efforts like continuous daily monitoring of representative haul-outs throughout the Wadden Sea are needed to elucidate this counterintuitive trend.
Since 2002, the abundance and distribution of harbour porpoises are assessed by annually conducting aerial surveys within German waters. This covers all the Natura2000 Special Areas of Conservation (SACs) as well as the most sensitive time, the birth period. Aerial surveys in the Dutch and Danish parts of the site are lacking, therefore these surveys should be extended to the entire Wadden Sea Area as well as the coastal North Sea waters of the Netherlands and Denmark. In the course of the internationally conducted SCANS surveys, all North Sea areas are covered (Hammond et al., 2002; Hammond et al., 2021).
Scientific fields where further research and monitoring is needed to improve the management and protection of the different species and their habitat are:
-
Elucidating the causes for still increasing harbour seal pup counts while moult counts are stagnating;
-
Identifying the habitat use at sea, by applying the most adequate techniques (telemetry, passive acoustic monitoring) to determine the dependence of the marine mammals on the use of specific areas in the Wadden Sea and in the North Sea;
-
Evaluating the effects of human activities on marine mammals including a) tourist activities in the Wadden Sea; b) underwater noise (from e.g. construction and shipping) in the feeding and haul-out habitats (the North Sea and the Wadden Sea).
-
Analysing the feeding ecology of marine mammals at a temporal and spatial scale enables the identification of inter-and intraspecific competition between marine mammal species, including the application of “state of the art” techniques. This will facilitate the understanding of the changes in the status of the populations, especially in the light of the growing anthropogenic use of their habitat and possible changes due to global warming;
-
Assessing the health status of marine mammals including the effects of diseases (including infectious agents) and contaminants, such as new pollutants by establishing or improving the stranding network (including collection, registration and dissection of deceased animals) as well as a health monitoring of living animals;
-
Improving the monitoring of population size and distribution of harbour porpoises in the Wadden Sea region a harmonizing of the spatial and temporal coverage of monitoring methods is needed. The parameters that are monitored should drive the decision on what methods are most suitable;
-
Evaluating the use of marine mammals as bio-indicators of environmental conditions.
Recommendation for management
Habitat fragmentation and loss
The North Sea and especially its coastal areas, which include the Wadden Sea, is characterized by high anthropogenic usage. This includes, among others, shipping, fisheries, tourism, sand extraction and offshore installations. In the process of marine spatial planning, areas are allocated to specific usages with certain priorities, fostering conflict between exploitation of the marine environment and conservation of ecologically important areas.
All three marine mammal species are protected under the EU Habitats Directive and are included as designated features in the Wadden Sea and adjacent conservation sites in the North Sea. It is required that within each site, the species should be kept in favourable conservation status. All three marine mammal species spend the majority of their time at sea (>80%) and as top predators, they are integral components of the food web in the Wadden Sea and the North Sea (Leopold et al., 2015a; de la Vega et al., 2016; Aarts et al., 2019; Damseaux et al., 2020). Their mobility allows them to utilise productive areas with high ecological value. For seals, connectivity between those productive areas and the sandbanks in the Wadden Sea (for resting, reproduction, moulting), is essential. For porpoises, the relations between areas are still to be determined. In marine spatial planning, conservation sites should be designated and managed to protect the species’ core areas, supporting systemic functions. This should include areas that function as migratory corridors allowing for adequate dispersal.
Anthropogenic activities, however, may be taking place adjacent to areas important for the marine mammals, crossing them or overlapping with them completely. This could result in habitat loss or fragmentation of habitats. Even impacts at some distance may impact the marine biota through for example underwater noise, pollution or light.
This highlights the importance of effective management for the conservation of species and habitats. Management measures should coordinate anthropogenic usage and minimise negative impacts on the protected species. As seals and harbour porpoises are highly mobile, it is and will be a challenge to coordinate and adapt human activities at sea according to the needs of these species. This is becoming even more critical given the growth of anthropogenic activities at sea, such as the massive extension of offshore wind energy. But also in the light of the resilience as the species will need to face natural changes such as global warming.
Within this context, it is recommended to start an ethical discussion as early as possible where the assets and drawbacks of these developments will be evaluated in-depth and recommendations be given on the level of protection that is still feasible for the three marine mammal species in times of an approaching climate crisis. In order to be able to have such discussions, it is crucial to have a solid understanding of the ecological basis of the respective species in turn highlighting the urging need to increase research efforts to assess basic ecological relationships and habitat use.
Noise pollution
Underwater noise pollution has increased during the last decades. Several regulations have been directed at reducing underwater noise, including the use of two indicators for underwater noise in describing “Good Environmental Status” (GES) under the Marine Strategy Framework Directive (MSFD). The MSFD recommends reducing the level of underwater noise by developing and implementing new technologies. This applies to ship engines and propulsion systems, oil and gas exploration techniques, pile-driving methods and others. For instance, it has been demonstrated that vibratory piling of anchor pipes may be less harmful for marine mammals and other sensitive organisms than traditional pile driving (Baltzer et al., 2020). Thus, regulatory authorities should promote these alternatives, if proven to be effective and less detrimental to the ecosystem. Furthermore, the use of bubble curtains around a point noise source, e.g. pile driving, seismic surveys and underwater explosions, also help to reduce high-frequency noise and should be mandatory to protect animals with a high-frequency hearing, as the harbour porpoise. This recommendation is not limited to the Wadden Sea.
Rehabilitation
In the Wadden Sea Area, rescue and rehabilitation of seals (i.e. the temporary rearing of a wild animal with the intent of releasing it back into its natural habitat) as a means of supporting the wild populations is not necessary as population numbers are high and well above the limit of 1,000 individuals that have been suggested to ensure a probability of persistence above 99 % over a 200-year period (Olsen et al., 2014). Instead, it may pose a threat to wild animals. This was declared in the Seal Agreement, Art. VI, 1 (1994) and as a consequence, these practices were forbidden in the following Seal Management Plans ever since, with some exceptions allowing for local interpretation.
This has led to variations between the Wadden Sea countries. In Denmark, the release of animals from captivity is forbidden, leading to a complete stop of rehabilitation efforts, while in Germany and the Netherlands several so-called seal centres have been or are active. In the Netherlands, where the numbers of rehabilitated seals have been extremely high (Brasseur, 2018), new regulations based on the “Advice of the Scientific Advisory Committee on Seal Rehabilitation in the Netherlands” (2018) were introduced in a Dutch “seal agreement”. These new regulations resulted in a significant change in practices and a drop in the number of seals rescued.
A dialogue integrating ethical, as well as management-related considerations, is needed, to discuss if any seal or, for example, only injured animals or pups orphaned due to direct anthropogenic activities should be brought in and treated in a rehabilitation centre. Also, the risks associated with the rehabilitation and release of seals originating from outside the Wadden Sea together with Wadden Sea animals should be further elaborated. It is particularly important to discuss whether the positive effects (higher chances of survival) outweigh negative effects of being held in captivity, such as stress, habituation to human presence as well as the exposure to pathogens affecting the individual and the wild population, along with a potential reduction of the natural process of biological selection affecting the population if performed continuously on a large scale.
Therefore, it is recommended to gain information on the success of rehabilitation. This should be done by independent researchers, rather than the seal centres themselves, by utilising GPS trackers to follow the fate and whereabouts of released individuals.
Further, it is recommended to establish a trilateral documentation system on which and how many seals are brought to and released from rehabilitation centres, the use of medication, especially antibiotics, as well as the documentation and evaluation of the occurrence and outbreaks of diseases and how many seals decrease during rehabilitation.
Fishery management
Fishery activities in areas overlapping with marine mammals may potentially result in disturbance, bycatch, collision, competition for fish and habitat destruction (Read, 2008). Sensitive habitats, such as reefs and sandbanks need to be protected from bottom trawling to keep their structure and function as spawning or feeding habitat for relevant prey fish for marine mammals. Furthermore, there is a need for reliable, processed and easily accessible data on fish stocks, fishing activities, marine mammal distribution, condition and diet. Collecting data on these aspects is essential to recognise deterioration early on to be able to act promptly. Areas of high density or of special importance (e.g. for feeding or breeding) of marine mammals should lead to the exclusion of industrial and gillnet fisheries at least within protected areas. This is necessary to avoid direct competition between fishery and marine mammals and the death of animals (e.g. in the whale sanctuary in the Schleswig-Holstein National Park) due to collisions or bycatch.
Health impacts
To assess the health status of the three Wadden Sea marine mammal populations, investigations of both dead and alive individuals are important. Therefore, stranding networks in all regions of the Wadden Sea need to be maintained, improved and, if not available, need to be established. A common protocol and a recommended minimum number of dead animals collected for post-mortem examinations from the regions every year is needed. These data should be combined in a common database to allow data treatment and evaluation on the trilateral level.
Good cooperation with the participating fishermen is needed to obtain bycaught marine mammals for post-mortem investigations. Information is also needed on bycatch rates in the Wadden Sea. This close cooperation should also be initiated for e.g. offshore constructions, shipping recreational and military activities to allow improved management and protection strategies. Governmental support is needed to acquire the necessary permits and to meet the requirements of several international conventions aiming to reduce human-induced mortality levels.
Chemical pollution, marine litter, prolonged disturbance from noise sources and climate change may result in indirect and chronic health effects, due to habitat degradation, changes in resource quantity and quality as well as increased risk of infectious disease outbreaks. It may therefore become necessary to expand the monitoring efforts to infectious agent screening, and target specific organs such as the auditory, endocrine, immune and reproductive systems. Parameter of these organ systems may be useful as an indicator for changes in the population caused by natural (e.g. interspecies activities, growing populations) or human-induced changes (e.g. climate change, offshore construction, fisheries, shipping). Moreover, monitoring schemes should be established in all areas of the Wadden Sea to assess the health of living animals.
Target health parameters of Wadden Sea harbour seals, harbour porpoises and grey seals will help to establish the Good Environmental Status (GES) as part of the European Marine Strategy Framework Directive (MSFD).
Other Human activities potentially affecting marine mammals and their prey
Several other human activities in and around the Wadden Sea potentially have an impact on marine mammals. This includes interferences due to recreational activities as well as activities such as the disposal of sludge or even sand extraction.
It is recommended to assess and monitor these activities and their potential interaction with marine mammals. This includes specifically:
-
as the number of seals hauled out in the Wadden Sea has grown, so have the outdoor activities close to the seal haul-out sites. This can potentially have negative impacts, especially during key life stages (like breeding and moulting). Guidelines for all outdoor activities, including kite surfing and organized seal safaris, should be formulated aiming to reduce disturbance from these activities;
-
for the continuous disposal of dredged material from numerous harbours and rivers bordering the Wadden Sea Area the actual impact on the ecosystem of the Wadden Sea is largely unclear. Analysis of the effects of dumping dredged material from Hamburg harbour has shown, increases of pollutant concentrations may occur and negative impacts are highly likely (Brockmeyer & Theobald, 2016). Dredging also affects the turbidity of the water which can have large impacts on the surrounding ecosystem.
5. Summary
The aim of the quality status report is to inform about the actual status of the harbour seal, grey seal and harbour porpoise within the Wadden Sea Area of Denmark, Germany and the Netherlands. As top predators, they are sentinels for ecosystem health. A variety of marine mammal experts of all three countries have evaluated and assessed the research data collected during the current reporting period to provide an overview of the status of the three species within the Wadden Sea region. For seals, research methods include surveys following established monitoring methods to provide information on abundance and distribution. For porpoises, information was collated from aerial survey work, passive acoustic monitoring, tagging, and stranding networks. Population biology and health parameters were collected during necropsies of stranded marine mammals. Monitoring methods and coverage differed between regions and countries. Area-wide and continuous monitoring are essential for being able to observe changes and possible negative developments and allow for timely measures for the conservation of the three marine mammal species within the Wadden Sea Area.
Marine mammals in the North Sea face a wide range of threats: underwater noise, fishing and pollution (contaminants and litter) which can affect their health and influence their distribution.
Despite the current anthropogenic impacts, harbour seal counts during moult revealed the highest numbers since the first assessment in 2000. The same is true for the grey seal population: here, a constant rise since their return to the Wadden Sea in the mid-20th century was observed. For harbour porpoises, a southward shift was detected during large scale SCANS surveys between 1994 and 2016. German aerial surveys revealed a decreasing trend in porpoise density in the Natura2000 site “Sylt Outer Reef” west of Sylt as well as a decline in the entire German North Sea. Telemetry data on six harbour porpoises indicated residency in the Wadden Sea waters.
Based on the current status of the harbour seal, grey seal and harbour porpoise, recommendations for the protection of these marine mammal species were formulated.
About the authorsUnger, B.1, Baltzer, J.1, Brackmann, J.4, Brasseur, S.3, Brügmann, M.4, Diederichs, B.5, Galatius, A.2, Geelhoed, S.C.V 3, Huus Petersen, H.9, IJsseldijk, L.L.8, Jensen, T. K.9, Jess, A.5, Nachtsheim, D.1, Philipp, C.1, Scheidat, M.7, Schop, J.3, Siebert, U.1, Teilmann, J.2, Thøstesen, C.B.6, van Neer, A. 1
1Institute for Terrestrial and Aquatic Wildlife Research (ITAW), University of Veterinary Medicine Hanover, Werftstr. 6, 25761 Büsum, DE 2Aarhus University, Marine Mammal Research, Department of Bioscience, Frederiksborgvej 399, 4000 Roskilde, DK 3 Wageningen Marine Research, Postbus 57, 1780 AB Den Helder, NL 4 Lower Saxony State Office for Consumer Protection and Food Safety, Food and Veterinary Institute Oldenburg, Martin-Niemöller-Str. 2, 26133 Oldenburg, DE 5 Schleswig-Holstein Agency for Coastal Defense, National Park and Marine Conservation, National Park Authority, Schlossgarten 1, 25832 Tönning, DE 6 Fiskeri- og Søfartsmuseet. Tarphagevej 2, DK-6710 Esbjerg, DK 7 Wageningen Marine Research, Wageningen University, Postbus 68, 1970 AB Ijmuiden, NL 8 Division of Pathology, Department of Biomolecular Health Sciences, Faculty of Veterinary Medicine, Utrecht University, Utrecht, NL 9 Center for Diagnostic, Technical University of Denmark, Kgs Lyngby, DK |
References
Aarts, G., Brasseur, S. and Kirkwood, R. (2018) Behavioural response of grey seals to pile-driving.
Aarts, G., Brasseur, S., Poos, J., Schop, J., Kirkwood, R., Van Kooten, T., Mul, E., Reijnders, P., Rijnsdorp, A. and Tulp, I. (2019) Top‐down pressure on a coastal ecosystem by harbor seals. Ecosphere 10(1): e02538.
Abt, K. (2010) Earlier pupping in harbour seals likely driven by climate – rather than by fisheries. Response to Reijnders P.J.H, Brasseur S.M.J.M. & Meesters E.H.W.G. Biology Letters 6: 854-857.
Ailsa, J., Bernie, J., McConnell, R.J.B. and Richard J, B. (2001) Factors affecting first‐year survival in grey seals and their implications for life history strategy. Journal of Animal Ecology 70(1): 138-149.
Alstrup, A. K. O., Thøstesen, C. B., Madsen, P. T., Petersen, H. H., Jensen, T. K., Olsen, M. T. and Kinze, C. C. (2021) First Stranding of Cuvier's Beaked Whale (Ziphius cavirostris) on the Danish North Sea Coast. Aquatic Mammals 47(3): 303-310.
ASCOBANS (2018) Review of New Information on Threats to Small Cetaceans (reporting cycle 2017 only) - 2017 Annual National Report: Germany. AC24/Inf.2.d.https://www.ascobans.org/sites/default/files/document/AC24_Inf_2.d_2017%20National%20Report_Germany.pdf.
Baer, J., Smaal, A., van der Reijden, K. and Nehls, G. (2017) Fisheries. Wadden Sea Quality Status Report 2017.qsr.waddensea-worldheritage.org/reports/fisheries.
Baltzer, J., Schaffeld, T., Ruser, A., Woelfing, B., Stührk, P. and Siebert, U. (2018) Akustisches Monitoring von Schweinswalen im Wattenmeer für den Landesbetrieb für Küstenschutz, Nationalpark und Meeresschutz Schleswig-Holstein und die Nationalparkverwaltung Niedersächsisches Wattenmeer 2018. Büsum, Germany.
Baltzer, J., Maurer, N., Schaffeld, T., Ruser, A., Schnitzler, J. G. and Siebert, U. (2020) Effect ranges of underwater noise from anchor vibration operations in the Wadden Sea. Journal of Sea Research 162: 101912.
Berges, B., Geelhoed, S., Scheidat, M. and Tougaard, J. (2019) Quantifying harbour porpoise foraging behaviour in CPOD data: identification, automatic detection and potential application.
BfN (2021) Fischereimanagement in Meeresschutzgebieten. https://www.bfn.de/fischereimanagement.
Boon, A., Caires, S., Wijnant, I., Verzijlbergh, R., Zijl, F., Schouten, J. and Kooten, T. (2018) The assessment of system effects of large-scale implementation of offshore wind in the southern North Sea. Report.
Bouveroux, T., Kiszka, J., Heithaus, R., Jauniaux, T. and Pezeryl, S. (2014) Direct evidence for gray seal (Halichoerus grypus) predation and scavenging on harbor porpoises (Phocoena phocoena). Marine Mammal Science.
Brandt, M. J., Dragon, A.-C., Diederichs, A., Bellmann, M. A., Wahl, V., Piper, W., Nabe-Nielsen, J. and Nehls, G. (2018) Disturbance of harbour porpoises during construction of the first seven offshore wind farms in Germany. Marine Ecology Progress Series 596: 213-232.
Brasseur, S. M., Polanen Petel, T. v., Aarts, G., Meesters, E., Dijkman, E. and Reijnders, R. (2009) Grey seals and Offshore Wind Farms.
Brasseur, S. M., Aarts, G., Rebolledo, E. B., Cremer, J., Fey-Hofstede, F., Geelhoed, S., Lindeboom, H. J., Lucke, K., Machiels, M. A. and Meesters, H. W. G. (2011) Zeezoogdieren in de Eems: studie naar de effecten van bouwactiviteiten van GSP, RWE en NUON in de Eemshaven in 2010 (herzien).
Brasseur, S. M., Aarts, G., Meesters, E., van Polanen Petel, T., Dijkman, E., Cremer, J. and Reijnders, P. (2012) Habitat preferences of harbour seals in the Dutch coastal area: analysis and estimate of effects of offshore wind farms. Report C043-10.
Brasseur, S. M., Aarts, G. and Kirkwood, R. (2014a) Habitat quality for grey seals in the Dutch Wadden Sea.
Brasseur, S. M., van Polanen Petel, T. D., Gerrodette, T., Meesters, E. H., Reijnders, P. J. and Aarts, G. (2014b) Rapid recovery of Dutch gray seal colonies fueled by immigration. Marine Mammal Science 31(2): 405-426.
Brasseur, S. M., van Polanen Petel, T. D., Gerrodette, T., Meesters, E. H., Reijnders, P. J. and Aarts, G. (2015) Rapid recovery of Dutch gray seal colonies fueled by immigration. Marine Mammal Science 31(2): 405-426.
Brasseur, S. M. (2017) Seals in motion - How movements drive population development of harbour seals and grey seals in the North Sea. Publisher. Pages.
Brasseur, S. M. (2018) Stranding and rehabilitation in numbers: population development and stranding data on the Dutch coasts 1990-2016: analysis of new data from a public database.
Brasseur, S. M., Reijnders, P. J., Cremer, J., Meesters, E., Kirkwood, R., Jensen, L. F., Jeβ, A., Galatius, A., Teilmann, J. and Aarts, G. (2018) Echoes from the past: regional variations in recovery within a harbour seal population. PloS one 13(1): e0189674.
Brasseur, S. M., Abel, C., Galatius, A., Jeß, A., Körber, P., Meise, K., Schop, J., Siebert, U., Teilmann, J., Thøstesen, C. B. and Klöpper, S. (2021) EG-Marine Mammals grey seal surveys in the Wadden Sea and Helgoland in 2020-2021.https://www.waddensea-worldheritage.org/sites/default/files/Wadden Sea_Grey_Seal_Report_2021_0.pdf.
Bravo Rebolledo, E. L. (2021) Afval in bruinvismagen Afval in bruinvismagen - Voor en na de MSC Zoë containerramp.
Brockmeyer, B. and Theobald, N. (2016) 20 Jahre Monitoring organischer Schadstoffe in Sedimenten der Deutschen Bucht: Zustand und zeitliche Entwicklung. Bundesamt für Seeschifffahrt und Hydrographie (BSH).
BSH (2021) GeoSeaPortal. Retrieved from https://www.geoseaportal.de.
Buckland, S. T., Anderson, D. R., Burnham, K. P., Laake, J. L., Borchers, D. L. and Thomas, L. (2001) Introduction to distance sampling estimating abundance of biological populations.
Camphuysen, C. (2004) The return of the harbour porpoise (Phocoena phocoena) in Dutch coastal waters. Lutra 47(2): 113-122.
Carstensen, J., Henriksen, O. and Teilmann, J. (2006) Impacts of offshore wind farm construction on harbour porpoises: acoustic monitoring of echolocation activity using porpoise detectors (T-PODs). Marine Ecology Progress Series 321: 295-308.
Common Wadden Sea Secretariat (2010) Wadden Sea Plan 2010 - Eleventh Trilateral Governmental Conference on the Protection of the Wadden Sea, C. W. S. Secretariat.
Cordes, L. S. and Thompson, P. M. (2013) Variation in breeding phenology provides insights into drivers of long-term population change in harbour seals. Proceedings of the Royal Society B: Biological Sciences 280(1764): 20130847.
Dähne, M., Gilles, A., Lucke, K., Peschko, V., Adler, S., Krügel, K., Sundermeyer, J. and Siebert, U. (2013) Effects of pile-driving on harbour porpoises (Phocoena phocoena) at the first offshore wind farm in Germany. Environmental Research Letters 8(2): 025002.
Damseaux, F., Siebert, U., Pomeroy, P., Lepoint, G. and Das, K. (2020) Habitat and resource segregation of two sympatric seals in the North Sea. Science of The Total Environment 764: 142842.
de la Vega, C., Lebreton, B., Siebert, U., Guillou, G., Das, K., Asmus, R. and Asmus, H. (2016) Seasonal variation of Harbor Seal's diet from the Wadden Sea in relation to prey availability. PloS one 11(5): e0155727.
Dietz, R., Teilmann, J., Andersen, S., Rigét, F. and Olsen, M. T. (2013) Movements and site fidelity of harbour seals (Phoca vitulina) in Kattegat, Denmark, with implications for the epidemiology of the phocine distemper virus. ICES Journal of Marine Science 70(1): 186-195.
FAO (1997) Fisheries Management.FAO Technical Guidelines for Responsible Fisheries Rome.http://www.fao.org/3/w4230e/w4230e.pdf.
Fietz, K., Galatius, A., Teilmann, J., Dietz, R., Frie, A. K., Klimova, A., Palsboll, P. J., Jensen, L. F., Graves, J. A., Hoffman, J. I. and Olsen, M. T. (2016) Shift of grey seal subspecies boundaries in response to climate, culling and conservation. Mol Ecol 25(17): 4097-112.https://www.ncbi.nlm.nih.gov/pubmed/27616353
https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/mec.13748?download=true.
Fontaine, M. C., Tolley, K. A., Michaux, J. R., Birkun Jr, A., Ferreira, M., Jauniaux, T., Llavona, A., Öztürk, B., Öztürk, A. A. and Ridoux, V. (2010) Genetic and historic evidence for climate-driven population fragmentation in a top cetacean predator: the harbour porpoises in European water. Proceedings of the Royal Society B: Biological Sciences 277(1695): 2829-2837.
Galatius, A., Brackmann, J., Brasseur, S. M., Diederichs, B., Jeß, A., Klöpper, S., Körber, P., Schop, J., Siebert, U., Teilmann, J., Thøstesen, B. and Schmidt, B. (2020a) Trilateral surveys of Harbour Seals in the Wadden Sea and Helgoland in 2020.
Galatius, A., Gilles, A., Ahola, M., Authier, M., Brasseur, S. and Geelhoed, S. (2020b) Working Group on Marine Mammal Ecology (WGMME). ICES.
Galatius, A., Brackmann, J., Brasseur, S. M., Diederichs, B., Jeß, A., Klöpper, S., Körber, P., Schop, J., Siebert, U., Teilmann, J., Thøstesen, B. and Schmidt, B. (2021) Trilateral surveys of Harbour Seals in the Wadden Sea and Helgoland in 2021.
Gilles, A., Andreasen, H., Müller, S. and Siebert, U. (2008) Nahrungsökologie von marinen Säugetieren und Seevögeln für das Management von NATURA 2000 Gebieten. Teil: Marine Säugetiere. Final report, submitted to the German Federal Agency for Nature Conservation (BfN). F+ E FKZ 805(85): 018.
Gilles, A., Scheidat, M. and Siebert, U. (2009) Seasonal distribution of harbour porpoises and possible interference of offshore wind farms in the German North Sea. Marine Ecology Progress Series 383: 295-307.https://www.int-res.com/articles/meps2009/383/m383p295.pdf.
Gilles, A., Viquerat, S., Becker, E., Forney, K., Geelhoed, S., Haelters, J., Nabe‐Nielsen, J., Scheidat, M., Siebert, U. and Sveegaard, S. (2016) Seasonal habitat‐based density models for a marine top predator, the harbor porpoise, in a dynamic environment. Ecosphere 7(6): e01367.
Goedknegt, M. A., Welsh, J. E., Drent, J. and Thieltges, D. W. (2015) Climate change and parasite transmission: how temperature affects parasite infectivity via predation on infective stages. Ecosphere 6(6): 1-9.
Greenwood, P. J. (1980) Mating systems, philopatry and dispersal in birds and mammals. Animal behaviour 28(4): 1140-1162.
Hammond, P., Berggren, P., Benke, H., Borchers, D., Collet, A., Heide‐Jørgensen, M., Heimlich, S., Hiby, A., Leopold, M. F. and Øien, N. (2002) Abundance of harbour porpoise and other cetaceans in the North Sea and adjacent waters. Journal of Applied Ecology 39(2): 361-376.
Hammond, P., Lacey, C., Gilles, A., Viquerat, S., Börjesson, P., Herr, H., Macleod, K., Ridoux, V., Santos, M. and Scheidat, M. (2017) Estimates of cetacean abundance in European Atlantic waters in summer 2016 from the SCANS-III aerial and shipboard surveys.
Hammond, P., Lacey, C., Gilles, A., Viquerat, S., Börjesson, P., Herr, H., Macleod, K., Ridoux, V., Santos, M. and Scheidat, M. (2021) Estimates of cetacean abundance in European Atlantic waters in summer 2016 from the SCANS-III aerial and shipboard surveys - Revised version.
Hammond, P. S., Macleod, K., Berggren, P., Borchers, D. L., Burt, L., Cañadas, A., Desportes, G., Donovan, G. P., Gilles, A., Gillespie, D., Gordon, J., Hiby, L., Kuklik, I., Leaper, R., Lehnert, K., Leopold, M., Lovell, P., Øien, N., Paxton, C. G. M., Ridoux, V., Rogan, E., Samarra, F., Scheidat, M., Sequeira, M., Siebert, U., Skov, H., Swift, R., Tasker, M. L., Teilmann, J., Van Canneyt, O. and Vázquez, J. A. (2013) Cetacean abundance and distribution in European Atlantic shelf waters to inform conservation and management. Biological Conservation 164: 107-122.
Hammond, P. S. and Wilson, L. (2016) Grey seal diet composition and prey consumption. Marine Scotland Science.
Härkönen, T. and Harding, K. (2001) Spatial structure of harbour seal populations and the implications thereof. Canadian Journal of Zoology 79(12): 2115-2127.
Härkönen, T., Dietz, R., Reijnders, P., Teilmann, J., Harding, K., Hall, A., Brasseur, S., Siebert, U., Goodman, S. J. and Jepson, P. D. (2006) The 1988 and 2002 phocine distemper virus epidemics in European harbour seals. Diseases of aquatic organisms 68(2): 115-130.
Härkönen, T., Brasseur, S., Teilmann, J., Vincent, C., Dietz, R., Abt, K. and Reijnders, P. (2007) Status of grey seals along mainland Europe from the Southwestern Baltic to France. NAMMCO Scientific Publications 6: 57-68.
Hasselmeier, I., Abt, K., Adelung, D. and Siebert, U. (2004) Stranding patterns of harbour porpoises (Phocoena phocoena) in the German North and Baltic Seas; when does the birth period occur. Journal of Cetacean Research and Management 6(3): 259-263.
Herr, H., Fock, H. O. and Siebert, U. (2009) Spatio-temporal associations between harbour porpoise Phocoena phocoena and specific fisheries in the German Bight. Biological Conservation 142(12): 2962-2972.
ICES (2020) Workshop on fisheries Emergency Measures to minimize BYCatch of short-beaked common dolphins in the Bay of Biscay and harbour porpoise in the Baltic Sea (WKEMBYC). ICES Scientific Reports 2(43).
IJsseldijk, L., Kik, M., Solé, L. and Gröne, A. (2017) Postmortaal onderzoek van bruinvissen (Phocoena phocoena) uit Nederlandse wateren, 2016.WOt-technical report 96.96.2352-2739.https://library.wur.nl/WebQuery/wurpubs/fulltext/418563.
IJsseldijk, L. L., ten Doeschate, M. T. I., Brownlow, A., Davison, N. J., Deaville, R., Galatius, A., Gilles, A., Haelters, J., Jepson, P. D., Keijl, G. O., Kinze, C. C., Olsen, M. T., Siebert, U., Thøstesen, C. B., van den Broek, J., Gröne, A. and Heesterbeek, H. (2020) Spatiotemporal mortality and demographic trends in a small cetacean: Strandings to inform conservation management. Biological Conservation 249: 108733.http://www.sciencedirect.com/science/article/pii/S0006320720307916.
IJsseldijk, L. L., Scheidat, M., Siemensma, M. L., Couperus, B., Leopold, M. F., Morell, M., Gröne, A. and Kik, M. J. (2021) Challenges in the Assessment of Bycatch: Postmortem Findings in Harbor Porpoises (Phocoena phocoena) Retrieved From Gillnets. Veterinary pathology 58(2): 405-415.
Jensen, L. F., Galatius, A. and Teilmann, J. (2015) First report on a newborn grey seal pup (Halichoerus grypus) in the Danish Wadden Sea since the 16th century. Marine Biodiversity Records 8.
Jensen, L. F., Teilmann, J., Galatius, A., Pund, R., Czeck, R., Jess, A., Siebert, U., Körber, P., & Brasseur, S. (2017). Wadden Sea Quality Status Report: Marine mammals. Common Wadden Sea Secretariat. https://doi.org/10.5281/zenodo.15197264
Jones, E. L., McConnell, B. J., Smout, S., Hammond, P. S., Duck, C. D., Morris, C. D., Thompson, D., Russell, D. J., Vincent, C. and Cronin, M. (2015) Patterns of space use in sympatric marine colonial predators reveal scales of spatial partitioning. Marine Ecology Progress Series 534: 235-249.
Kabat, P., Bazelmans, J., van Dijk, J., Herman, P. M., van Oijen, T., Pejrup, M., Reise, K., Speelman, H. and Wolff, W. J. (2012) The Wadden Sea Region: Towards a science for sustainable development. Ocean & Coastal Management 68: 4-17.
Kastelein, R. A., Helder-Hoek, L., Covi, J. and Gransier, R. (2016) Pile driving playback sounds and temporary threshold shift in harbor porpoises (Phocoena phocoena): Effect of exposure duration. The Journal of the Acoustical Society of America 139(5): 2842-2851.
Kesselring, T., Viquerat, S., Brehm, R. and Siebert, U. (2017) Coming of age: Do female harbour porpoises (Phocoena phocoena) from the North Sea and Baltic Sea have sufficient time to reproduce in a human influenced environment? PloS one 12(10): e0186951.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5650184/pdf/pone.0186951.pdf.
Kinze, C. C., Czeck, R., Herr, H. and Siebert, U. (2021) Cetacean strandings along the German North Sea coastline 1604–2017. Journal of the Marine Biological Association of the United Kingdom: 1-20.
Lakemeyer, J., Lehnert, K., Woelfing, B., Pawliczka, I., Silts, M., Dähne, M., von Vietinghoff, V., Wohlsein, P. and Siebert, U. (2020) Pathological findings in North Sea and Baltic grey seal and harbour seal intestines associated with acanthocephalan infections. Diseases of aquatic organisms 138: 97-110.
Lambert, E., MacLeod, C. D., Hall, K., Brereton, T., Dunn, T. E., Wall, D., Jepson, P. D., Deaville, R. and Pierce, G. J. (2011) Quantifying likely cetacean range shifts in response to global climatic change: implications for conservation strategies in a changing world. Endangered Species Research 15(3): 205-222.
Learmonth, J. A., MacLeod, C. D., Santos, M. B., Pierce, G. J., Crick, H. and Robinson, R. (2006) Potential effects of climate change on marine mammals. Oceanography and Marine Biology 44: 431.
Leopold, M. F. (2015) Eat and be eaten: Porpoise diet studies. Publisher. Pages.
Leopold, M. F., Begeman, L., Heße, E., van der Hiele, J., Hiemstra, S., Keijl, G., Meesters, E. H., Mielke, L., Verheyen, D. and Gröne, A. (2015a) Porpoises: from predators to prey. Journal of Sea Research 97: 14-23.
Leopold, M. F., Begeman, L., van Bleijswijk, J. D., IJsseldijk, L. L., Witte, H. J. and Gröne, A. (2015b) Exposing the grey seal as a major predator of harbour porpoises. Proceedings of the Royal Society B: Biological Sciences 282(1798): 20142429.
Lodder, Q. J., Wang, Z. B., Elias, E. P., van der Spek, A. J., de Looff, H. and Townend, I. H. (2019) Future Response of the Wadden Sea Tidal Basins to Relative Sea-Level rise—An Aggregated Modelling Approach. Water 11(10): 2198.
Lucke, K., Siebert, U., Lepper, P. A. and Blanchet, M.-A. (2009) Temporary shift in masked hearing thresholds in a harbor porpoise (Phocoena phocoena) after exposure to seismic airgun stimuli. The Journal of the Acoustical Society of America 125(6): 4060-4070.
Ludes-Wehrmeister, E., Wohlsein, P., Prenger-Berninghoff, E., Ewers, C., Woelfing, B., Lehnert, K. and Siebert, U. (2020) Intestinal displacements in older harbour and grey seals. Diseases of aquatic organisms 138: 215-225.
MacDonald, A., Speirs, D. C., Greenstreet, S. P., Boulcott, P. and Heath, M. R. (2019) Trends in sandeel growth and abundance off the east coast of Scotland. Frontiers in Marine Science 6: 201.
MacLeod, C. D., Santos, M. B., Reid, R. J., Scott, B. E. and Pierce, G. J. (2007) Linking sandeel consumption and the likelihood of starvation in harbour porpoises in the Scottish North Sea: could climate change mean more starving porpoises? Biology letters 3(2): 185-188.
MacLeod, C. D. (2009) Global climate change, range changes and potential implications for the conservation of marine cetaceans: a review and synthesis. Endangered Species Research 7(2): 125-136.
McConnell, B., Fedak, M., Lovell, P. and Hammond, P. (1999) Movements and foraging areas of grey seals in the North Sea. Journal of Applied Ecology 36(4): 573-590.
Meyer, J., Kroencke, I., Bartholomae, A., Dippner, J. W. and Schueckel, U. (2016) Long-term changes in species composition of demersal fish and epibenthic species in the Jade area (German Wadden Sea/Southern North Sea) since 1972. Estuarine, Coastal and Shelf Science 181: 284-293.
Mikkelsen, L., Johnson, M., Wisniewska, D. M., van Neer, A., Siebert, U., Madsen, P. T. and Teilmann, J. (2019) Long‐term sound and movement recording tags to study natural behavior and reaction to ship noise of seals. Ecology and evolution 9(5): 2588-2601.
Nabe‐Nielsen, J., van Beest, F. M., Grimm, V., Sibly, R. M., Teilmann, J. and Thompson, P. M. (2018) Predicting the impacts of anthropogenic disturbances on marine populations. Conservation Letters 11(5): e12563.
Nachtsheim, D. A., Viquerat, S., Ramírez-Martínez, N. C., Unger, B., Siebert, U. and Gilles, A. (2021) Small cetacean in a human high-use area: Trends in harbour porpoise abundance in the North Sea over two decades. Frontiers in Marine Science 7: 1135.
Nunny, L. and Simmonds, M. P. (2019) Climate Change and Cetaceans–an update.
Olsen, M. T., Andersen, L. W., Dietz, R., Teilmann, J., Härkönen, T. and Siegismund, H. R. (2014) Integrating genetic data and population viability analyses for the identification of harbour seal (Phoca vitulina) populations and management units. Molecular Ecology 23(4): 815-831.
Orians, G. H. and Pearson, N. E. (1979) On the theory of central place foraging. Analysis of ecological systems: 155-177.
Osinga, N., Pen, I., De Haes, H. U. and Brakefield, P. M. (2012) Evidence for a progressively earlier pupping season of the common seal (Phoca vitulina) in the Wadden Sea. Marine Biological Association of the United Kingdom. Journal of the Marine Biological Association of the United Kingdom 92(8): 1663.
OSPAR (2021) Proposal for a threshold for common indicator M6 (marine mammal bycatch).Agenda Item 4.3, BDC 21/4/7 Add3, draft.
Perrin, W. F., Würsig, B. and Thewissen, J. (2009) Encyclopedia of marine mammals. Academic Press.
Peschko, V., Müller, S., Schwemmer, P., Mercker, M., Lienau, P., Rosenberger, T., Sundermeyer, J. and Garthe, S. (2020) Wide dispersal of recently weaned grey seal pups in the Southern North Sea. ICES Journal of Marine Science 77(5): 1762-1771.
Philipp, C., Unger, B., Ehlers, S. M., Koop, J. H. and Siebert, U. (2021) First evidence of retrospective findings of microplastics in harbour porpoises (Phocoena phocoena) from German waters. Frontiers in Marine Science 8: 508.
Poulin, R. and Mouritsen, K. (2006) Climate change, parasitism and the structure of intertidal ecosystems. Journal of helminthology 80(2): 183-191.
Prummel, W. and Heinrich, D. (2005) Archaeological evidence of former occurrence and changes in fishes, amphibians, birds, mammals and molluscs in the Wadden Sea area. Helgoland Marine Research 59(1): 55-70.
Ransijn, J. M., Both, C. and Smout, S. C. (2019) A calorific map of harbour porpoise prey in the North Sea, JNCC. No. 633.
Read, A. J. (2008) The looming crisis: interactions between marine mammals and fisheries. Journal of Mammalogy 89(3): 541-548.
Reijnders, P. (1981) Management and conservation of the harbour seal, Phoca vitulina, population in the international Wadden Sea area. Biological Conservation 19(3): 213-221.
Reijnders, P. (1983) The effect of seal hunting in Germany on the further existence of a harbour seal population in the Dutch Wadden Sea. Zeitschrift Für Säugetierkunde 48(1981): 50-54.
Reijnders, P., van Dijk, J. and Kuiper, D. (1995) Recolonization of the Dutch Wadden Sea by the grey seal Halichoerus grypus. Biological Conservation 71(3): 231-235.
Reijnders, P., Ries, E. H., Tougaard, S., Nørgaard, N., Heidemann, G., Schwarz, J., Vareschi, E. and Traut, I. M. (1997) Population development of harbour seals Phoca vitulina in the Wadden Sea after the 1988 virus epizootic. Journal of Sea Research 38(1-2): 161-168.
Reijnders, P., Brasseur, S. and Brinkman, A. (2003) The phocine distemper virus outbreak of 2002 amongst harbour seals in the North Sea and Baltic Sea: spatial and temporal development, and predicted population consequences. Management of North Sea harbour and grey seal populations: proceedings of the International Symposium at EcoMare, Texel, The Netherlands November 29-30, 2002. Common Wadden Sea Secretariat [etc.]. 19-25.
Reijnders, P., Brasseur, S., Abt, K., Siebert, U., Stede, M. and Tougaard, S. (2006) Aerial Surveys of Harbour and Grey Seals in the Wadden Sea in 2006.Wadden Sea Newsletter 2006, T. S. E. G. (TSEG): 2.https://www.waddensea-worldheritage.org/sites/default/files/2006_Harbour%20seal%20report.pdf.
Reijnders, P., Brasseur, S. M. J. M., T. Borchardt, , Camphuysen, K., Czeck, R., Gilles, A., Jensen, J. F., Leopold, M., K. Lucke, K., Ramdohr, S., Scheidat, M., Siebert, U. and Teilmann, J. (2009) Marine Mammals.Thematic Report T. M. a. A. Group. No. 25. No. 20.qsr.waddensea-worldheritage.org/reports/marine-mammals.
Reijnders, P., Brasseur, S. M. and Meesters, E. H. (2010a) Earlier pupping in harbour seals, Phoca vitulina. Biology letters 6(6): 854-857.
Reijnders, P., Brasseur, S. M., Tougaard, S., Seibert, U., Borchardt, T. and Stede, M. (2010b) Population development and status of harbour seals (Phoca vitulina) in the Wadden Sea. NAMMCO Scientific Publications 8: 95-105.
Reise, K. and van Beusekom, J. E. (2008) Interactive effects of global and regional change on a coastal ecosystem. Helgoland Marine Research 62(1): 85-91.
Ridoux, V., Konigson, S., Macleod, K., Authier, M., Brasseur, S. M. (2020) Workshop on fisheries Emergency Measures to minimize BYCatch of short-beaked common dolphins in the Bay of Biscay and harbour porpoise in the Baltic Sea (WKEMBYC), I. S. Reports. No. 43.https://doi.org/10.17895/ices.pub.7472.
Rohner, S., Hülskötter, K., Gross, S., Wohlsein, P., Abdulmawjood, A., Plötz, M., Verspohl, J., Haas, L. and Siebert, U. (2020) Male grey seal commits fatal sexual interaction with adult female harbour seals in the German Wadden Sea. Scientific reports 10(1): 1-11.
Russell, D. J., McClintock, B. T., Matthiopoulos, J., Thompson, P. M., Thompson, D., Hammond, P. S., Jones, E. L., MacKenzie, M. L., Moss, S. and McConnell, B. J. (2015) Intrinsic and extrinsic drivers of activity budgets in sympatric grey and harbour seals. Oikos 124(11): 1462-1472.
Russell, D. J., Hastie, G. D., Thompson, D., Janik, V. M., Hammond, P. S., Scott‐Hayward, L. A., Matthiopoulos, J., Jones, E. L. and McConnell, B. J. (2016) Avoidance of wind farms by harbour seals is limited to pile driving activities. Journal of Applied Ecology 53(6): 1642-1652.
Santos, M., Pierce, G. J., Learmonth, J. A., Reid, R., Ross, H., Patterson, I., Reid, D. and Beare, D. (2004) Variability in the diet of harbor porpoises (Phocoena phocoena) in Scottish waters 1992–2003. Marine Mammal Science 20(1): 1-27.
Schade, F. M., Raupach, M. J. and Wegner, K. M. (2016) Seasonal variation in parasite infection patterns of marine fish species from the Northern Wadden Sea in relation to interannual temperature fluctuations. Journal of Sea Research 113: 73-84.
Scheidat, M., Gilles, A., Kock, K.-H. and Siebert, U. (2008) Harbour porpoise Phocoena phocoena abundance in the southwestern Baltic Sea. Endangered species research 5(2-3): 215-223.
Scheidat, M., Tougaard, J., Brasseur, S., Carstensen, J., van Polanen Petel, T., Teilmann, J. and Reijnders, P. (2011) Harbour porpoises (Phocoena phocoena) and wind farms: a case study in the Dutch North Sea. Environmental Research Letters 6(2): 025102.
Scheidat, M., Verdaat, H. and Aarts, G. (2012) Using aerial surveys to estimate density and distribution of harbour porpoises in Dutch waters. Journal of Sea Research 69: 1-7.
Scheidat, M., Teilmann, J., Baltzer, J., Thøstesen, C., Diederichs, B., Dietz., R., Geelhoed, S. C. V., Gilles, A., IJsseldijk, L. L., Keijl, G. O., Nabe-Nielsen, J., Ruser, A., Schnitzler, J., Sveegaard, S., Vrooman, J. and Siebert, U. (under review) Status of harbour porpoise in the Wadden Sea World Heritage Site and requirements for trilateral monitoring. Marine Biology.
Schop, J., Meijboom, A. and Brasseur, S. (2020) MSC ZOE: mogelijke effecten van de verloren containers op zeehonden.
Schulte, K., Schulze, T. and Kraus, G. (2015) Wissen bündeln für ein nachhaltiges Management der Krabbenfischerei im Küstenmeer einschließlich der Wattenmeer Nationalparks (MaKramee) : Projektabschlussbericht: 100.
Schuster, E., Bulling, L. and Köppel, J. (2015) Consolidating the state of knowledge: a synoptical review of wind energy’s wildlife effects. Environmental management 56(2): 300-331.
SCOS (2019) Scientific advice on matters related to the management of seal populations: 2019: 211.http://www.smru.st-andrews.ac.uk/files/2016/08/SCOS-2015.pdf.
Sell, A., Pusch, C., von Dorrien, C., Krause, J., Schulze, T. and Carstensen, D. (2011) Maßnahmenvorschläge für das Fischereimanagement in Natura 2000-Gebieten der deutschen AWZ der Nord-und Ostsee. Bundesamt für Naturschutz, Johann-Heinrich-von-Thünen-Institut, Leibniz-Institut für Geowissenschaften IFM-GEOMAR. Vilm, Germany.
Sharples, R. J., Arrizabalaga, B. and Hammond, P. S. (2009) Seals, sandeels and salmon: diet of harbour seals in St. Andrews Bay and the Tay Estuary, southeast Scotland. Marine Ecology Progress Series 390: 265-276.
Siebert, U., Wunschmann, A., Weiss, R., Frank, H., Benke, H. and Frese, K. (2001) Post-mortem findings in harbour porpoises (Phocoena phocoena) from the German North and Baltic Seas. Journal of Comparative Pathology 124(2-3): 102-14.http://www.ncbi.nlm.nih.gov/pubmed/11222006.
Siebert, U., Gilles, A., Lucke, K., Ludwig, M., Benke, H., Kock, K.-H. and Scheidat, M. (2006) A decade of harbour porpoise occurrence in German waters - analyses of aerial surveys, incidental sightings and strandings. Journal of Sea Research 56(1): 65-80.
Siebert, U., Prenger‐Berninghoff, E. and Weiss, R. (2009) Regional differences in bacterial flora in harbour porpoises from the North Atlantic: environmental effects? Journal of Applied Microbiology 106(1): 329-337.
Siebert, U., Müller, S., Gilles, A., Sundermeyer, J. and Narberhaus, I. (2012) Species Profiles marine mammals. Threatened biodiversity in the German North and Baltic Seas - Sensitivities towards human activities and the effects of climate change. Narberhaus I., Krause J. and B. J. Bonn - Bad Godesberg. No. 116: 487-542.
Siebert, U., Pawliczka, I., Benke, H., von Vietinghoff, V., Wolf, P., Pilāts, V., Kesselring, T., Lehnert, K., Prenger-Berninghoff, E. and Galatius, A. (2020) Health assessment of harbour porpoises (Phocoena phocoena) from Baltic area of Denmark, Germany, Poland and Latvia. Environment international 143: 105904.
Sills, J. M., Ruscher, B., Nichols, R., Southall, B. L. and Reichmuth, C. (2020) Evaluating temporary threshold shift onset levels for impulsive noise in seals. The Journal of the Acoustical Society of America 148(5): 2973-2986.
Simmonds, M. P. and Eliott, W. J. (2009) Climate change and cetaceans: concerns and recent developments. Journal of the Marine Biological Association of the United Kingdom 89(1): 203-210.
Sonne, C., Lakemeyer, J., Desforges, J.-P., Eulaers, I., Persson, S., Stokholm, I., Galatius, A., Gross, S., Gonnsen, K. and Lehnert, K. (2020) A review of pathogens in selected Baltic Sea indicator species. Environment international 137: 105565.
Sørensen, T. B. and Kinze, C. C. (1994) Reproduction and reproductive seasonality in Danish harbour porpoises, Phocoena phocoena. Ophelia 39(3): 159-176.
Stalder, D., van Beest, F. M., Sveegaard, S., Dietz, R., Teilmann, J. and Nabe-Nielsen, J. (2020) Influence of environmental variability on harbour porpoise movement. Marine Ecology Progress Series 648: 207-219.
Sveegaard, S., Teilmann, J., Tougaard, J., Dietz, R., Mouritsen, K. N., Desportes, G. and Siebert, U. (2011) High‐density areas for harbor porpoises (Phocoena phocoena) identified by satellite tracking. Marine Mammal Science 27(1): 230-246.
Sveegaard, S., Galatius, A., Kyhn, L. A. and Teilmann, J. (2019) Havpattedyr - sæler og marsvin in Marine områder 2018. NOVANA. J. W. Hansen and S. Høgslund. Aarhus, Denmark: Aarhus University, DCE – Danish Centre for Environment and Energy. 87-99.
Teilmann, J., Larsen, F. and Desportes, G. (2007) Time allocation and diving behaviour of harbour porpoises (Phocoena phocoena) in Danish and adjacent waters. J Cetacean Res Manag 9: 201-210.
Thøstesen, C. B., Uldal, C., Teilmann, J., Olsen, M. T., Kinze, C. C. and Hansen, M. S. (2016) Strandede Havpattedyr i Danmark 2015 & 2016 - Beredskabet vedrørende Havpattedyr (Status report), T. F. a. M. Museum.
Thøstesen, C. B., Uldal, C., Teilmann, J., Olsen, M. T., Kinze, C. C. and Hansen, M. S. (2017) Strandede Havpattedyr i Danmark 2017 - Beredskabet vedrørende Havpattedyr (Status report), T. F. a. M. Museum.
Thøstesen, C. B., Uldal, C., Teilmann, J., Olsen, M. T., Kinze, C. C. and Hansen, M. S. (2018) Strandede Havpattedyr I Danmark 2018, Beredskabet vedrørende Havpattedyr (Status report), T. F. a. M. Museum.
Thøstesen, C. B., Uldal, C., Teilmann, J., Olsen, M. T., Kinze, C. C. and Hansen, M. S. (in prep.) Strandede Havpattedyr i Danmark 2019 - Beredskabet vedrørende Havpattedyr (Status report), T. F. a. M. Museum.
Tougaard, J., Teilmann, J. and Tougaard, S. (2008) Harbour seal spatial distribution estimated from Argos satellite telemetry: overcoming positioning errors. Endangered species research 4(1-2): 113-122.
Tougaard, J., Carstensen, J., Teilmann, J., Skov, H. and Rasmussen, P. (2009) Pile driving zone of responsiveness extends beyond 20 km for harbor porpoises (Phocoena phocoena (L.)). The Journal of the Acoustical Society of America 126(1): 11-14.
Tulp, I., Bolle, L. J. and Rijnsdorp, A. D. (2008) Signals from the shallows: In search of common patterns in long-term trends in Dutch estuarine and coastal fish. Journal of Sea Research 60(1-2): 54-73.
van Beest, F. M., Teilmann, J., Dietz, R., Galatius, A., Mikkelsen, L., Stalder, D., Sveegaard, S. and Nabe-Nielsen, J. (2018) Environmental drivers of harbour porpoise fine-scale movements. Marine Biology 165(5): 95.
Van Bressem, M.-F., Raga, J. A., Di Guardo, G., Jepson, P. D., Duignan, P. J., Siebert, U., Barrett, T., de Oliveira Santos, M. C., Moreno, I. B. and Siciliano, S. (2009) Emerging infectious diseases in cetaceans worldwide and the possible role of environmental stressors. Diseases of aquatic organisms 86(2): 143-157.https://www.int-res.com/articles/dao2009/86/d086p143.pdf.
van der Veer, H. W., Dapper, R., Henderson, P. A., Jung, A. S., Philippart, C. J., Witte, J. I. and Zuur, A. F. (2015) Changes over 50 years in fish fauna of a temperate coastal sea: Degradation of trophic structure and nursery function. Estuarine, Coastal and Shelf Science 155: 156-166.
van Franeker, J. A., Rebolledo, E. L. B., Hesse, E., IJsseldijk, L. L., Kühn, S., Leopold, M. and Mielke, L. (2018) Plastic ingestion by harbour porpoises Phocoena phocoena in the Netherlands: Establishing a standardised method. Ambio: 1-11.
van Neer, A., Gross, S., Kesselring, T., Grilo, M. L., Ludes-Wehrmeister, E., Roncon, G. and Siebert, U. (2020) Assessing harbour porpoise carcasses potentially subjected to grey seal predation. Scientific Reports 10(1): 16345.https://doi.org/10.1038/s41598-020-73258-y.
van Neer, A., Gross, S., Kesselring, T., Grilo, M. L., Ludes-Wehrmeister, E., Roncon, G. and Siebert, U. (2021) Assessing seal carcasses potentially subjected to grey seal predation. Scientific reports 11(1): 1-9.
Van Walraven, L., Dapper, R., Nauw, J. J., Tulp, I., Witte, J. I. and van der Veer, H. W. (2017) Long-term patterns in fish phenology in the western Dutch Wadden Sea in relation to climate change. Journal of Sea Research 127: 173-181.
Vance, H., Hooker, S., Mikkelsen, L., van Neer, A., Teilmann, J., Siebert, U. and Johnson, M. (2021) Drivers and constraints on offshore foraging in harbour seals. Scientific reports 11(1): 1-14.
Weel, S. M., Geelhoed, S. C., Tulp, I. and Scheidat, M. (2018) Feeding behaviour of harbour porpoises (Phocoena phocoena) in the Ems estuary. Lutra 61: 137-152.
Wenger, D. and Koschinski, S. (2012) Harbour porpoise (Phocoena phocoena Linnaeus, 1758) entering the Weser river after decades of absence. Marine Biology Research 8(8): 737-745.
WGBYC (2020) Working Group on Bycatch of Protected Species (WGBYC).ICES Scientific Reports https://doi.org/10.17895/ices.pub.7471.
Whyte, K. F., Russell, D. J., Sparling, C. E., Binnerts, B. and Hastie, G. D. (2020) Estimating the effects of pile driving sounds on seals: Pitfalls and possibilities. The Journal of the Acoustical Society of America 147(6): 3948-3958.
Wilson, L. J. and Hammond, P. S. (2019) The diet of harbour and grey seals around Britain: Examining the role of prey as a potential cause of harbour seal declines. Aquatic Conservation: Marine and Freshwater Ecosystems 29: 71-85.
Wilson, R. P., Liebsch, N., Gomez-Laich, A., Kay, W. P., Bone, A., Hobson, V. J. and Siebert, U. (2015) Options for modulating intra-specific competition in colonial pinnipeds: the case of harbour seals (Phoca vitulina) in the Wadden Sea. PeerJ 3: e957.
Wisniewska, D. M., Johnson, M., Teilmann, J., Siebert, U., Galatius, A., Dietz, R. and Madsen, P. T. (2018) High rates of vessel noise disrupt foraging in wild harbour porpoises (Phocoena phocoena). Proceedings of the Royal Society B: Biological Sciences 285(1872): 20172314.
Woth, K., Weisse, R. and Von Storch, H. (2006) Climate change and North Sea storm surge extremes: an ensemble study of storm surge extremes expected in a changed climate projected by four different regional climate models. Ocean Dynamics 56(1): 3-15.
Zein, B., Woelfing, B., Dähne, M., Schaffeld, T., Ludwig, S., Rye, J. H., Baltzer, J., Ruser, A. and Siebert, U. (2019) Time and tide: Seasonal, diel and tidal rhythms in Wadden Sea Harbour porpoises (Phocoena phocoena). PLOS ONE 14(3): e0213348.https://doi.org/10.1371/journal.pone.0213348.
This report should be cited as: Unger, B., Baltzer, J., Brackmann, J., Brasseur, S., Brügmann, M., Diederichs, B., Galatius, A., Geelhoed, S., Huus Petersen, H., IJsseldijk, L., Jensen, T. K., Jess, A., Nachtsheim, D. A., Philipp, C., Scheidat, M., Schop, J., Siebert, U., Teilmann, J., Thøstesen, C. B., & van Neer, A. (2022). Wadden Sea Quality Status Report: Marine mammals. Common Wadden Sea Secretariat. https://doi.org/10.5281/zenodo.15223944
All 2022 reports may be cited collectively as: Kloepper, S., Bostelmann, A., Bregnballe, T., Busch, J.A., Buschbaum, C., Deen, K., Domnick, A., Gutow, L., Jensen, K., Jepsen, N., Luna, S., Meise, K., Teilmann, J., & van Wezel, A. (2022). Wadden Sea Quality Status Report. Common Wadden Sea Secretariat, Wilhelmshaven, Germany. Downloaded DD.MM.YYYY. qsr.waddensea-worldheritage.org